Fungicide Mode-of-Action: The LabCoat Guide to Pesticides & BioPesticides
In this tenth article in the LabCoat Guide to Pesticide Mode of Action, I discuss Fungicide Mode-of-Action.
(This article originally appeared on BioScience Solution´s AGBIOSCIENCE & BIOSOLUTIONS BLOG – please consider subscribing to receive similar articles).
Fungicides are synthetic or natural chemical compounds or biological organisms able to kill or inhibit fungi or fungal spore germination, as well as oomycetes. This is achieved through several different Modes of Action (MOA).
The chemical structure of the fungicide active ingredient (AI) usually defines its mode of action by determining its uptake and systemicity, and its ability to reach and bind with its target site – the physical location where the fungicide acts.
A sound knowledge of fungal biology and fungicidal mode of action is important for determining how to optimally use a fungicide, and for understanding the underlying mechanisms of resistance and selectivity.
Fungal Biology
True fungi (Kingdom Mycota) share several biochemical or structural features, which are the targets of fungicides and which may impart specificity i.e. the fungicide kills or inhibits fungi. The fungicidal target Chitin is a component of fungal and insect cell walls, and Ergosterol is a component of fungal cell membranes, but is absent in animal and plant cell membranes. Ergosterol is formed in fungi via a multistep ergosterol synthesis pathway – a factor which has important implications for resistance management.
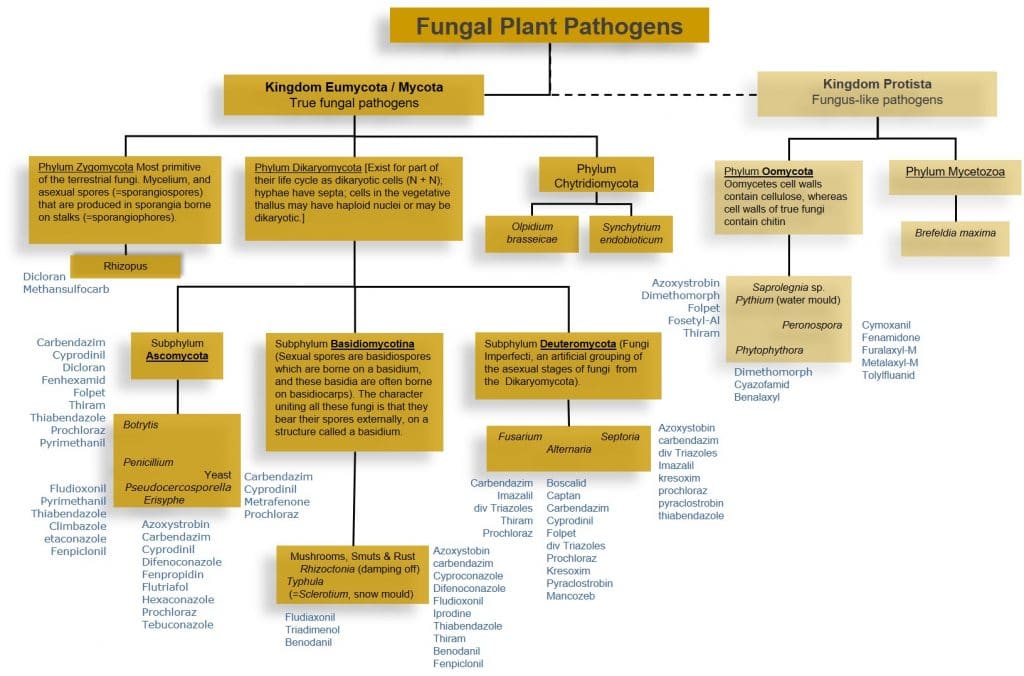
Figure 1: Classification of major fungal and oomycetes plant pathogens.
The Oomycetes (Kingdom Protista) are not true fungi, but use the same mechanisms and strategies to infect plants. Plant pathologists group then with fungal pathogens. Oomycetes include destructive plant pathogens including the genus Phytophthora (potato late blight), Pythium (damping off disease) and Peronospora / Pseudoperonospora (Downy Mildews). Instead of chitin, oomycetes cell walls contain cellulose, and ergosterol is not a principle sterol in the Oomycete cell membrane. Fungicides targeting chitin and ergosterol synthesis are thus generally not effective against these pathogens. Downy mildews can be confused with the unrelated fungal powdery mildews, but are insensitive to fungicides able to control powdery mildew.
Mode of Action
In the following, fungicides and their modes of action are classified into three broad groups:
- Inhibitors of sterol synthesis.
- Inhibitors of mitochondrial electron transport (respiration inhibitors).
- Multi-site enzyme inhibitors, nucleic acid and protein synthesis inhibitors.
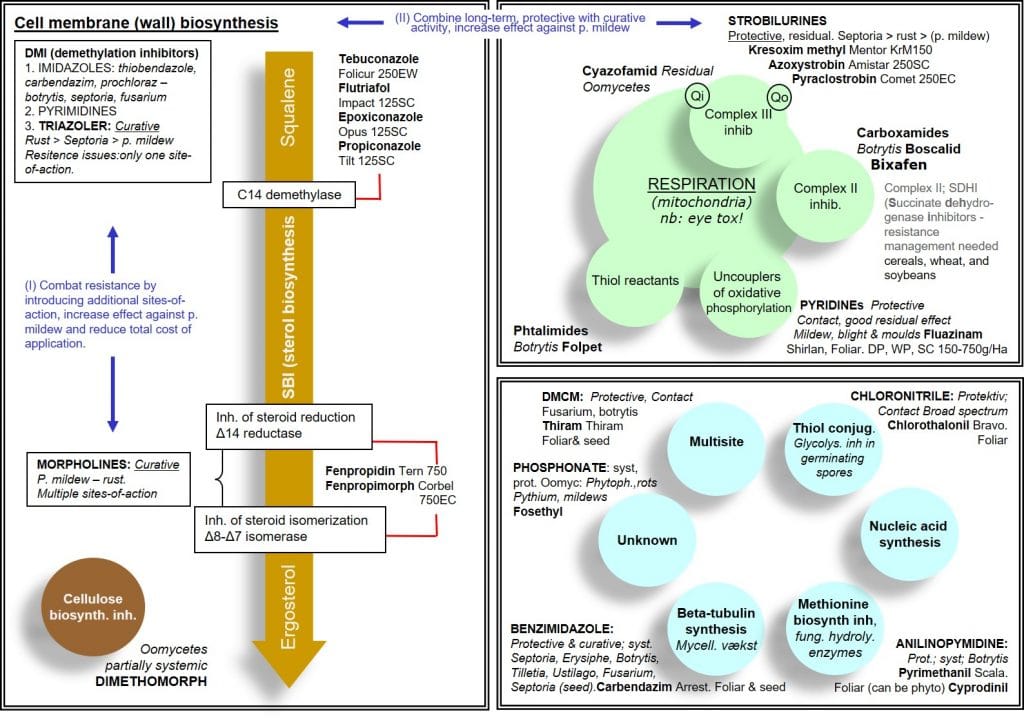
Figure 2: Summary of key fungicides and their modes of action.
Inhibitors of ergosterol synthesis (cell membrane synthesis and integrity)
Because ergosterol is a component of fungal cell membranes, but absent in animal and plant cell membranes, its synthesis is an important target for fungicides. Ergosterol is formed from its precursor squalene via a multistep ergosterol synthesis pathway, and is necessary for membrane structure, integrity and function.
Demethylation inhibitors (DMIs) are a group of systemic fungicides targeting cell membrane integrity through the inhibition of C14-demethylation during ergosterol biosynthesis. DMIs include Imidazoles, Pyrimidines and Triazoles with primary primarily curative activity. As this group of fungicides targets a single metabolic site, the risk of resistance is high unless they are used with other fungicides having different modes of action. Their primary efficacy is against Basidiomycetes (rust pathogens), followed by Deuteromycetes (Septoria) and to a lesser extent Ascomycetes (Powdery Mildews).
A second group of ergosterol synthesis inhibitors are the morpholines, which act by inhibiting the sterol reductase and sterol isomerase enzymes of the ergosterol synthesis pathway. This group of fungicides has curative systemic activity and – in contrast to DMIs – is effective against Ascomycetes. By having multiple sites of action, the risk of resistance development is low for this group.
Morpholines can be used in tank or formulated mixtures with DMIs, broadening the spectrum of activity and reducing the risk of resistance. Morpholines tend to be less costly than DMIs, and combinations of these products can reduce the overall cost of pathogen control.
Although both dimethomorph and fenpropimorph are classified as morpholines, the former is an inhibitor of cellulose synthase and acts against Oomycetes whereas the latter is an inhibitor of ergosterol biosynthesis and acts against foliar Ascomycetes.
Inhibitors of mitochondrial electron transport (respiration inhibitors)
The second major group of fungicides are inhibitors of mitochondrial respiration. As mitochondrial respiration is a common metabolic process across all organisms, inhibition of respiration may lead to problems with selectivity, i.e. they could be toxic to non-target organisms. However, most fungicides in this group show selective toxicity towards fungi, in part due to selective uptake and translocation.
Strobilurins form part of the group of quinone-outside inhibitor (QoI) fungicides, inhibiting mitochondrial respiration at the Qo site of cytochrome b, part of the cytochrome bc1 complex (Complex III), preventing spore germination and mycelial growth in fungal pathogens.
Strobilurin fungicides can be both partially systemic and protectant in action. They are active against most cereal fungal diseases, including septoria, rusts and mildew.
As mitochondrial respiration also occurs in Oomycetes, this group of fungicides may also control pathogens such as Plasmopara viticola, responsible for downy mildew. Strobilurins also have physiological benefits, maintaining green leaf area for longer (termed ‘greening’) and delaying crop senescence.
Strobilurins are generally as effective as triazoles, although triazoles are more systemic and have greater curative activity. Strobilurins have a great residual long-term activity, and can be used in tank or formulated mixtures with triazoles, broadening the spectrum of activity and permitting greater flexibility of spray timing.
Also inhibiting mitochondrial respiration at Complex Ⅲ, Cyazofamid is an Oomycetes-selective quinone-inside inhibitor (QiI) fungicide.

Figure 3: Modes of action of mitochondrial respiration inhibitors.
Carboxamides form part of the group of succinate dehydrogenase inhibitors (SDHIs), inhibiting the activity of mitochondrial Complex II (succinate dehydrogenase) and thus respiration in fungal cells. As Complex II is a common mitochondrial enzyme system found in eukaryotic and prokaryotic organisms, non-target effects of complex II inhibitors must be considered.
The Pyridine group of fungicides are proton gradient uncouplers, inhibiting mitochondrial respiration in fungal pathogens by uncoupling the proton gradient and reducing the proton driven synthesis of ATP via the enzyme ATP synthase.
Folpet and the phthalimide fungicide Captan are thiol reactants, blocking the activity of thiol-containing enzymes involved in mitochondrial respiration in fungal spores. Their protective action is mainly due to inhibition of spore germination. As for many other respiration inhibitors, they are also active against certain oomycetes, such as Pythium.
Others: multi-site enzyme inhibitors, nucleic acid and protein synthesis inhibitors.
Multi-site enzyme inhibitors such as Chlorothalonil and Thiram inhibit several thiol-containing enzymes involved in respiration, while single site fungicides, such as epoxiconazole and azoxystrobin only inhibit a single, specific metabolic process.
As pathogens struggle to overcome multiple metabolic processes, the risk of resistance is low for multi-site fungicides. Besides multi-site fungicides, risk management programs are based on the use of multiple active ingredients with different modes of action.
Nucleic acid metabolism and protein synthesis inhibitors include benzimidazoles, phenylamides and dicarboximides, and inhibit DNA as well as RNA synthesis, affecting cell division and cellular metabolism.
Fungicide systemicity
Fungicide uptake and distribution in leaves and plants (systemicity) depends on the lipophilicity or hydrophilicity (determined by logP and pKa) of their constituent active ingredients.
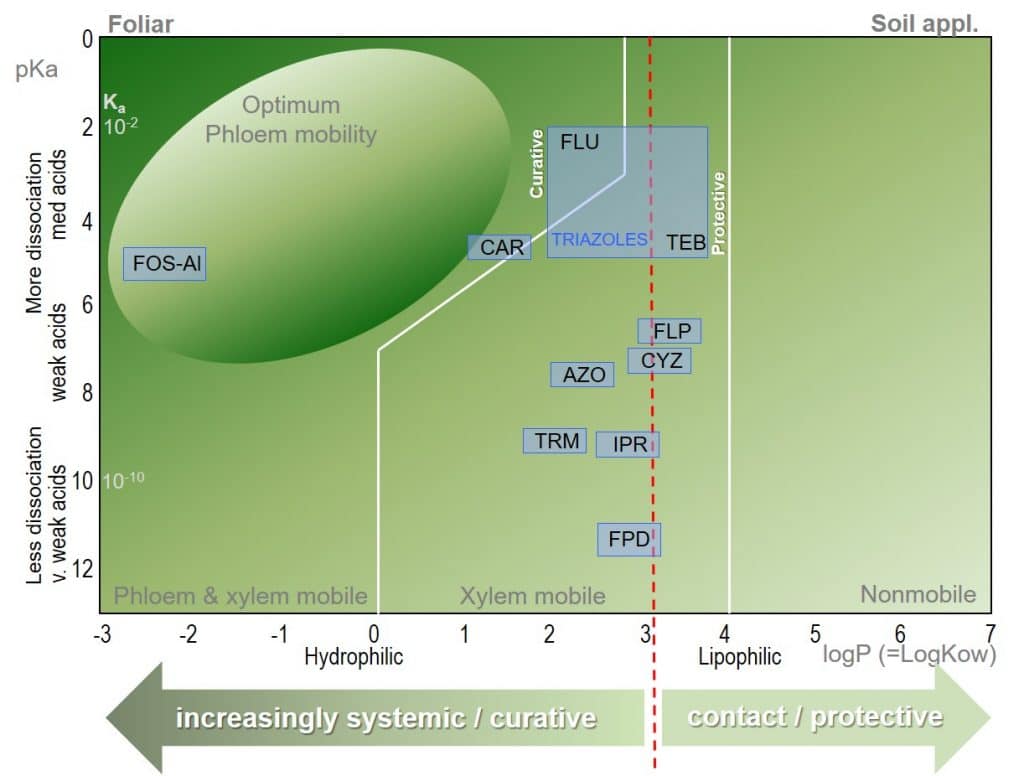
Figure 4: Bromilow model for the prediction of pesticide mobility in plants for common fungicides.
The partition coefficient (logP) and dissociation constant (pKa) of a fungicidal active ingredient are determinants of their mobility in plants. The logP of a fungicide molecule describes its lipophilicity or hydrophilicity, where lipophilicity describes a molecule’s ability to dissolve in lipophilic (non-aqueous) solutions, allowing permeation through biological membranes.
The pKa value of a fungicide molecule defines the pH at which it is neutral. At greater pH values, acid groups will be charged, while at lower pH values base groups will be charged. The number and distribution of charges on a molecule affect its aqueous solubility.
Many common fungicides can be seen to be clustered around the “low” end of the systemicity scale, and are primarily xylem mobile. For triazoles, systemicity may be relatively low e.g. Tebuconazole, TEB; logP=3.7, pKa=5.0) while Flutriafol (FLU; logP=2.3, pKa=2.3) is considered to be the most systemic triazole.
Here, the Bromilow model demonstrates its value for plant physiologists: although the Triazoles lie within a relatively narrow interval in the Bromilow model, their mobility ranges from systemic to relatively non-systemic, and care should be taken before recommending Triazole fungicides as curative treatments.
Protective and curative modes of action
For a fungicide to be considered curative, it is necessary for the active ingredient to be systemically translocated within the plant, to enable it to inhibit (cure) existing fungal hyphal growth within the leaf.
Curative fungicides maybe applied shortly after infection, although it should be noted that their efficacy declines once the disease is established in the field. They are systemic and can be absorbed into the leaf and translocated via the xylem within the leaf (locally systemic) or the plant (acropetal systemicity). Besides controlling pathogens which have already entered the plant, systemic or curative fungicides have longer residual activity as they are protected from wash-off and weathering. Due to their systemic nature, curative fungicides can reach plant tissues difficult to reach using spray equipment.
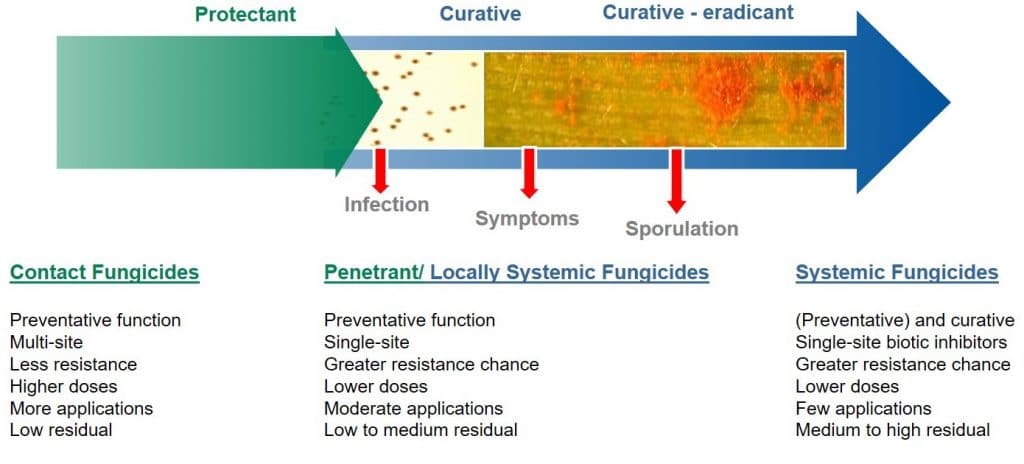
Figure 5: Application timing for contact (protectant) versus systemic (curative) fungicides.
In contrast, protective (protectant) fungicides are described as having a contact action (but may exhibit some systemic activity), remaining on the surface of the leaf where they can protect the plant against the germination of newly-landed fungal spores. Protective fungicides require application before infection by the pathogen takes place.
Translaminar fungicides (locally systemic), such as QoI or strobilurin fungicides redistribute the fungicide from the upper, sprayed leaf surface to the lower, unsprayed surface. Translaminar movement takes place via the lipophilic leaf surface and lipophilic extra cellular pathways. Some Triazole (protective and early-curative) fungicides may also have translaminar activity.
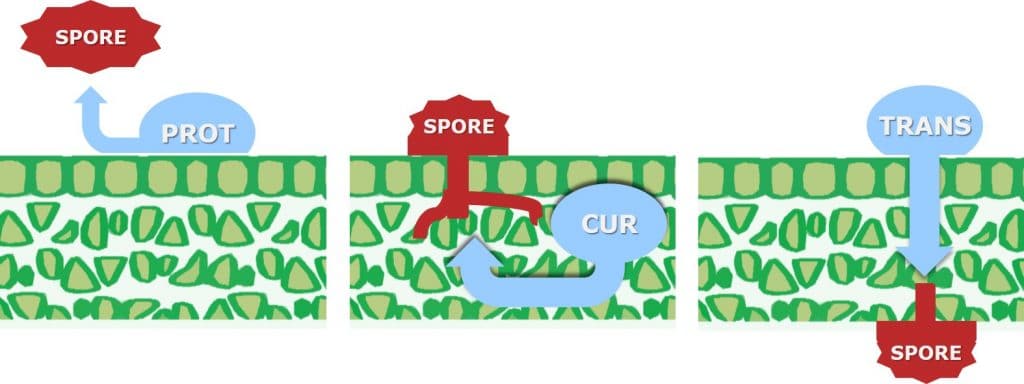
Figure 6: Leaf uptake and movement for contact (protective) and systemic (curative and translaminar) fungicides.
Fungicide uptake and translocation
By affecting uptake across the cuticula and intracellular or extracellular transport within the plant, adjuvants are an additional factor deciding whether chemical and biochemical active ingredients have apoplastic (extracellular), symplastic (intracellular) or translaminar (from adaxial to abaxial leaf surface) activity.
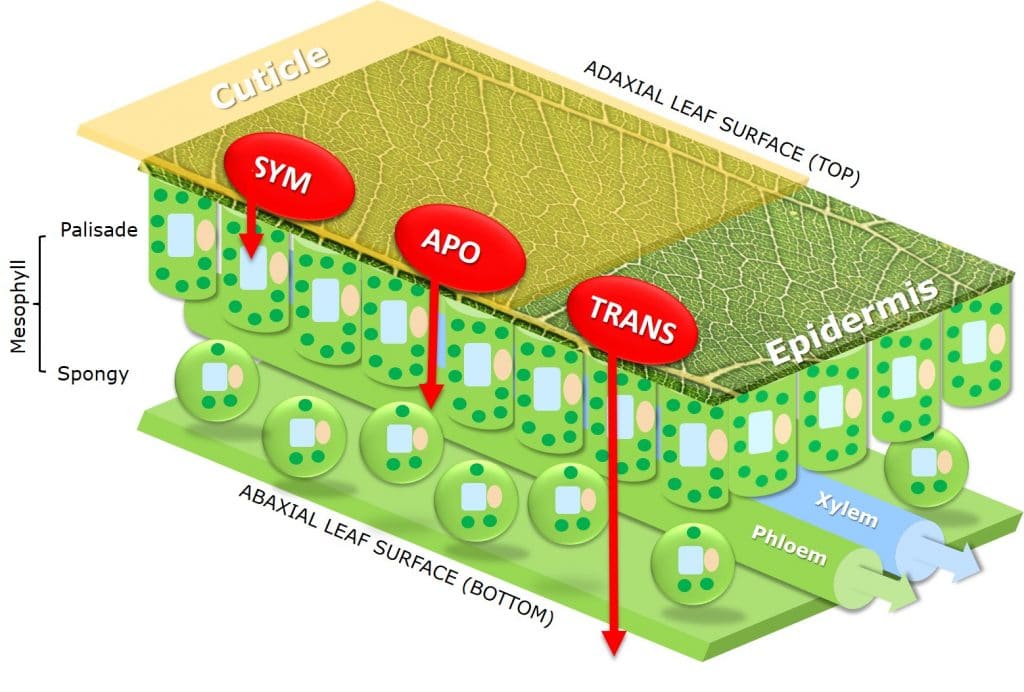
Figure 7: Apoplastic (extracellular), symplastic (intracellular) or translaminar (from adaxial to abaxial leaf surface) active ingredient pathways.
Biofungicide mode of action
Biofungicides are formulations of living organisms or natural metabolites used to control plant pathogenic fungi. Many successful microbial biofungicides can colonise and grow in treated soil or on plant leaves, providing a long-term source of microbial and biochemical modes of action.
Biofungicide strategies depend on multiple factors, including the efficacy of microbial metabolites, the induction of localized or systemic plant defence responses, parasitization of (or competition with) other fungi and plant growth stimulation, and may provide a viable alternative or supplement to conventional crop protection strategies.
For a detailed breakdown of biofungicide mode of action, please refer to the article in the LabCoat Guide series titled: Biopesticide Mode of Action – Biofungicides.
***
Thanks for reading – if you liked what you read, please consider liking and sharing. Your feedback is invaluable, and helps new readers discover my work!
Please feel free to read and share my other articles in this series:

A little about myself
I am a Plant Scientist with a background in Molecular Plant Biology and Crop Protection.
20 years ago, I worked at Copenhagen University and the University of Adelaide on plant responses to biotic and abiotic stress in crops.
At that time, biology-based crop protection strategies had not taken off commercially, so I transitioned to conventional (chemical) crop protection R&D at Cheminova, later FMC.
During this period, public opinion as well as increasing regulatory requirements gradually closed the door of opportunity for conventional crop protection strategies, while the biological crop protection technology I had contributed to earlier began to reach commercial viability.
From January 2018, I will be available to provide independent Strategic R&D Management as well as Scientific Development and Regulatory support to AgChem & BioScience organizations developing science-based products.
For more information, visit BIOSCIENCE SOLUTIONS – Strategic R&D Management Consultancy
Keywords: #cropprotection #biologicals #biopesticides #pesticides #formulation #plantscience #agchem #agbio #bioscience #authorpreneur #projectmanagement #biological #biopesticide #pesticide #R&D #strategy #consultant #strategic #management #executive #insead #srdm
***



December 17, 2017 @ 9:09 pm
How copper, sulfur and zinc act as fungicides. Fantastic article.
Thanks.
March 7, 2019 @ 4:18 am
very good information about fungicides mode of action- would you please send these information in PDF TO my e-mail – mhrashwan@gmail.com
October 12, 2020 @ 11:34 am
remarkable. It is a fantastic job.
I would like to see more
Congratulations
July 3, 2021 @ 6:41 am
Can I use your illustrations to make YouTube videos for educational purpose? I find them very useful. I will give due acknowledgement in my presentation.
Regards
July 8, 2021 @ 5:55 pm
Absolutely – some may have been revised, feel free to reach out.
December 1, 2021 @ 7:33 am
Great article with lots of important information regarding bio fungicide. Thanks for writing this informative article and helping many gardeners with their doubts about how we can use fungicide in gardens or farms. Thanks again
January 5, 2022 @ 8:17 am
Great effort . Good information . Could you send these knowledge Bank to shankarkbiotech@gmail.com
April 19, 2022 @ 7:42 pm
Thanks for this valuable information about mode of action of fungicides.
I’d appreciate having this chapter in my email