The LabCoat Guide to Pesticide Mode of Action: Herbicide Safeners
In this fifth article in the LabCoat Guide to Pesticide Mode of Action, I discuss Herbicide Safeners and their Biochemical Mode of Activity.
A little about my background
I am a plant scientist with a background in Molecular Plant Biology and Crop Protection. 20 years ago, I worked at Copenhagen University on photosynthetic responses to stress in crops. Subsequently, I worked in Australia on molecular defense mechanisms induced by Phylloxera attack in grapevine.
At that time, biology-based crop protection strategies had not taken off commercially, so I transitioned to conventional (chemical) crop protection R&D at Cheminova, later FMC.
During this period, public opinion, as well as increasing regulatory requirements, gradually closed the door of opportunity for conventional crop protection strategies, while the biological crop protection technology I had contributed to earlier began to reach commercial viability.
From January 2018, I will consolidate 17+ years of industry experience in BioScience R&D management with my academic research background, to provide independent Strategic R&D Management as well as Scientific Development and Regulatory support to AgChem & BioScience organizations developing science-based products.
For more information, visit BIOSCIENCE SOLUTIONS – Strategic R&D Management Consultancy.
***
In the previous article in this series, I discussed the importance of a sound knowledge of herbicide mode of action for determining the optimal use of herbicides, as well as for understanding the underlying mechanisms of weed resistance and herbicide selectivity.
Herbicide safeners protect crops from herbicide injury by accelerating the metabolism of herbicides, or by inhibiting their translocation within plants, facilitating selectivity between crop plants and weed species targeted by the herbicide. Safeners may be applied to crop seeds before planting, or may be applied directly to plants as a mixture with the herbicide.
Herbicide safeners are typically used in monocotyledonous (grass) crops such as cereals, maize, rice and sorghum, and have permitted the development of cereal grass-weed herbicides, in which herbicides are able to target grass weeds without damaging grass (cereal) crops.
Herbicide safening
Herbicide safening is illustrated in figure 1, which shows the response of barley seedlings to the application of a selective grass herbicide in the presence (left) and absence (right) of a safener. The safener acts as a catalyst promoting the metabolization of herbicidal compounds in the cereal crop.

Figure 1: Grass-herbicide treated cereal plants in the presence (left) and absence (right) of a herbicide safener.
Safeners can reduce adverse herbicidal effects on the crops such as shoot and root growth inhibition, as well as delayed ear development:

Figure 2: The herbicidal effect of a safened and unsafened grass herbicide on cereals: delayed ear development in the absence of safeners (top, right) and inhibited shoot and root growth (bottom, right) is partially remediated by the inclusion of a safener (bottom, middle and top, left). Untreated plants included for comparison (bottom, left).
Grass herbicides of the aryloxyphenoxy group such as Fenoxaprop and Clodinafop target specific enzymes catalysing the biosynthesis of fatty acids, thereby inhibiting the synthesis and maintenance of organic plant constituents, such as cell and chloroplast (thylakoid) membranes.
Mechanisms of herbicide safening
In the following figure, the enzyme (in green) responsible for fatty acid biosynthesis functions normally, catalyzing the conversion of precursors to fatty acids.

Figure 3: Fatty acid biosynthesis in plants.
The herbicide Fenoxaprop inhibits the enzymatic process of fatty acid biosynthesis (Figure 4) by specifically binding to the ACCase (acetyl-CoA carboxylase) enzyme. This binding is dependent on compatibility between the three-dimensional binding site on the enzyme, and the three-dimensional shape of the herbicide molecule.
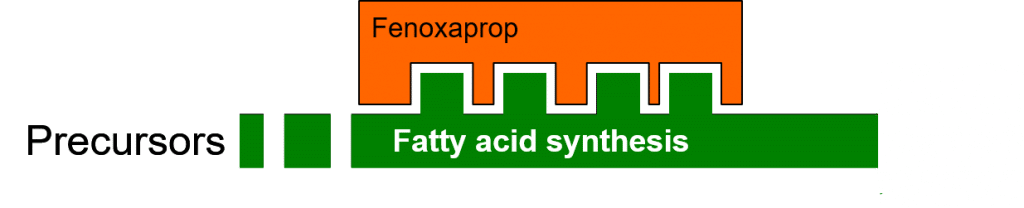
Figure 4: Herbicidal inhibition of fatty acid biosynthesis.
The addition of a safener may increase the synthesis of the naturally-occurring glutathione molecule in treated cereals, through its effect on the activity of the enzyme glutathione-S-transferase (GST). One of the functions of glutathione in plants is to conjugate with foreign compounds (such as xenobiotics) in the first step toward their detoxification and elimination, e.g. through partitioning of the conjugated xenobiotic into vacuoles.
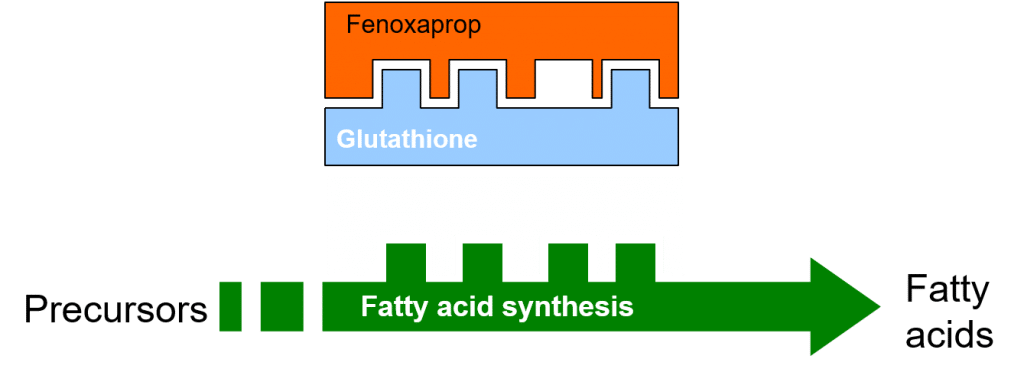
Figure 5: Increased glutathione synthesis and glutathione conjugation with Fenoxaprop, following safener induction.
Accordingly, the increased concentration of glutathione in safener-treated cereal crops permits increased levels of glutathione conjugation and detoxification of the herbicide.
Herbicide safening is species-specific
Natural glutathione levels differ between cereal crop species, and species-dependent differences in safener-induced glutathione concentrations provide differing levels of safening in these crops. In the following figure, levels of safening increase from Oat through Barley to Wheat.

Figure 6: Species-dependent safening in different cereal crop species.
Herbicide safening is herbicide-specific
Herbicides safeners are specific to herbicide classes and – as they act on metabolic processes – also to crop species. In cereals, safeners target glutathione conjugation and herbicide metabolism (de-esterification). The most common grass weed herbicides used in cereals are the Aryloxyphenoxy herbicides Fenoxaprop and Clodinafop, as well as Sulphonylurea (SU) herbicides. The herbicide safener Cloquintocet increases the activity of GST in cereals, leading to increased glutathione conjugation and detoxification of Fenoxaprop and Clodinafop, while the safener Mefenpyr increases the glutathione conjugation of Fenoxaprop and SUs.
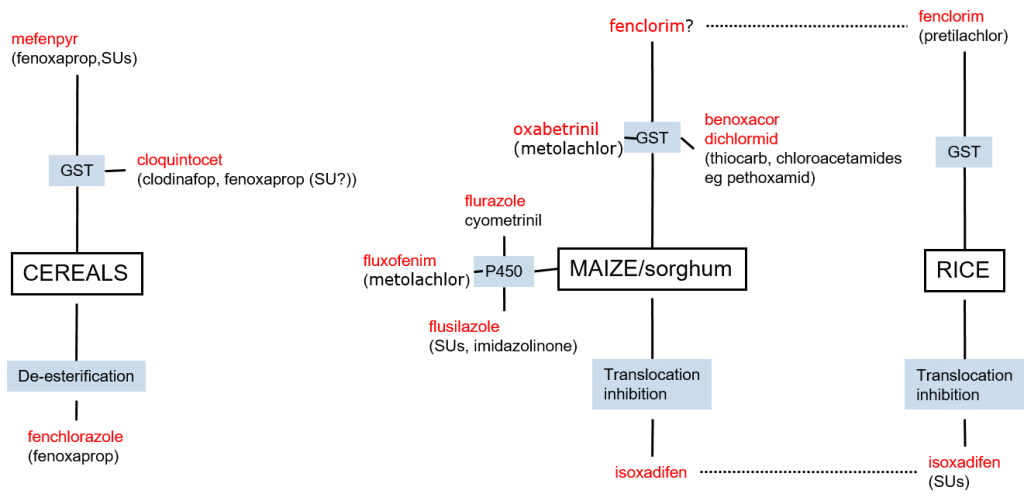
Figure 6
7: Schematic overview of herbicide safeners (red) across species and safener mechanisms (blue).
In cereal crops, the safener Fenchlorazole mediates the de-esterification of Fenoxaprop-p-ethyl to its phytotoxic form, Fenoxaprop, as well as its subsequent metabolism to non-toxic metabolites.
In maize and sorghum, safening maybe mediated through glutathione conjugation, through the activity of cytochrome P450 enzymes, as well as through translocation inhibition. Importantly, it may be seen that herbicide safeners which are effective in cereals are not effective in maize and sorghum. For research purposes, it is vital to perform experiments on relevant crops, and not to assume that safeners appropriate for one species are applicable to another. And yes, I am speaking from experience!
In contrast to safener specificity between cereals and e.g. maize, there is an overlap between safeners appropriate for maize and rice. Thus, herbicide safening in rice may be mediated by the same mechanisms and safeners of glutathione conjugation and translocation inhibition. Notably, however, herbicide safening mediated by P450 metabolism appears to be absent in rice.
Current Developments
Herbicides safeners are an effective method of improving herbicide selectivity, and have allowed the development of grass-weed herbicides for use in grass crops such as cereals, maize and rice. Current developments include the identification and development of safeners for use in dicotyledonous crops. For example, there is evidence that insecticides may act as herbicide safeners in soya, and safener activity has been observed in crops such as potatoes and oilseed rape. It is fair to assume that these safener mechanisms will not be the same as those for dicotyledonous crops.
***
Thanks for reading – please help make the BioScience Blog great by liking or sharing this article, making it visible to your network!
Harry Teicher is the founder of BIOSCIENCE SOLUTIONS and an Authorpreneur, providing organizations with Strategic- and Project Management as well as Development & Communication solutions. Follow him on Linkedin, Twitter and Facebook.