BioPesticide Mode-of-Action: The LabCoat Guide to Pesticides & BioPesticides
In this final article in the LabCoat Guide to Pesticide Mode of Action, I discuss Fungicide Mode-of-Action.
(This article originally appeared on BioScience Solution´s AGBIOSCIENCE & BIOSOLUTIONS BLOG – please consider subscribing to receive similar articles).
BioPesticides are pesticides based on microorganisms or natural products. Biopesticides introduce unique and complex modes of action, and can be used in plant production on an equal footing with chemical pesticides – if used correctly.
Most biopesticides are intended for use with traditional pesticides, which has contributed to the rapid increase in their use – the global market for chemical pesticides is experiencing annual growth rates of about 15%, while the global biopesticide market has an annual growth rate of about 5%.
Biopesticides can increase crop yields by working synergistically with chemical pesticides to optimize crop protection strategies, by allowing timely reuptake intervals and through their ability to be used close to harvest. Biopesticide demand is thus also being driven by growers increased knowledge about the mechanisms of action of biopesticides and how to use them.
A key issue regarding the efficiency of biopesticides and field conditions is that they are affected by weather and pest pressure, and require considerable insight regarding mode of action, application timing and placement of the formulated product.
This article summarizes the different classifications in biopesticides and discusses their respective ways of action from a physiological and biochemical point of view. The aim is to provide a clearer understanding of biopesticides and how they work.
BioHerbicide mode of action
Bioherbicides include microorganisms that infect weeds, and natural compounds with herbicidal activity sourced from microorganisms or plants. Bioherbicides target many of the same plant metabolic processes targeted by conventional herbicides, but they may bind differently to enzymes, or they may have multiple modes of action, making them potentially useful for treating herbicide-resistant weeds. Bioherbicides fall into two major classes:
• Microbial bioherbicides
• Bio-derived (biochemical) bioherbicides
Microbial bioherbicides include bacterial and fungal endophytes (microorganisms which colonize plants without causing disease) as well as viruses.
The modes of action of fungal bioherbicides (mycoherbicides) include the action of plant cell wall degrading enzymes, photobleaching molecules (macrocidins) and phytotoxic molecules such as the diterpene chenopodolin, and growth-regulating plant hormones such as indole acetic acid, IAA. Phoma macrostoma is an example of a mycoherbicide developed for pre-emergence weed control.
Bacterial bioherbicides exhibit phytotoxic activity attributed to specific metabolites as well as the elicitation of plant defense responses (see below) such as programmed cell death through the induction of reactive oxygen species..
Bio-derived (biochemical) bioherbicides (phytotoxins) are based on mechanisms used by microbes and plants to exclude invasive plant species from their immediate vicinity.
Allelopathy is a mechanism by which plants produce one or more biochemicals (allelochemicals) that affect the growth and survival of other plants.
The study of allelopathy has, among others, led to the identification of leptospermone, a herbicidal allelochemical produced by the Lemon Bottlebrush, and the subsequent development of its commercial chemical analogue, Mesotrione (Callisto).
Biochemical bioherbicides target many of the same plant metabolic processes targeted by conventional (synthetic) herbicides. However, natural phytotoxins may bind to proteins at different sites than their synthetic counterparts, or may have multiple modes of action. For example, sorgoleone (from Sorghum bicolor), a D1-protein-binding inhibitor of photosystem II can control weeds resistant to the QB-site PSII-inhibitor, atrazine.
Besides its inhibition of PSII electron transport and the concomitant increase in reactive oxygen species (ROS), sorgoleone inhibits the enzymatic activity of thylakoidal HPPD (hydroxyphenylpyruvate dioxygenase) and thus the formation of vitamin E, deriving the plant of one of its primary antioxidant protective mechanisms. Finally, sorgoleone inhibits mitochondrial respiration by blocking proton-gradient-driven ATP synthesis.
Chloroplast-based metabolic processes inhibited by natural phytotoxins include photosynthetic electron transport, chlorophyll and carotenoid biosynthesis, amino acid biosynthesis as well as lipid membrane function and stability.
Sorgoleone (Sorghum bicolor) and stigmatellin (from Stigmatella aurantica) are inhibitors of photosystem II (and hydroxyphenylpyruvate dioxygenase, HPPD), while pyridazocidin (Streptomyces spp.) is an inhibitor of photosystem I. Aurachins (Stigmatella aurantica) inhibit electron flow between PSII and PSI (Figure 1).
In photosynthesis, the photolysis of water leads to an accumulation of protons in the thylakoids stroma which drive the synthesis of ATP (photophosphorylation) via the enzyme ATP synthase (ATPase). Nigericin (Streptomyces) uncouples the proton gradient, inhibiting photophosphorylation while tentoxin (Alternaria tenuis) blocks photon flow through the chloroplast ATPase.
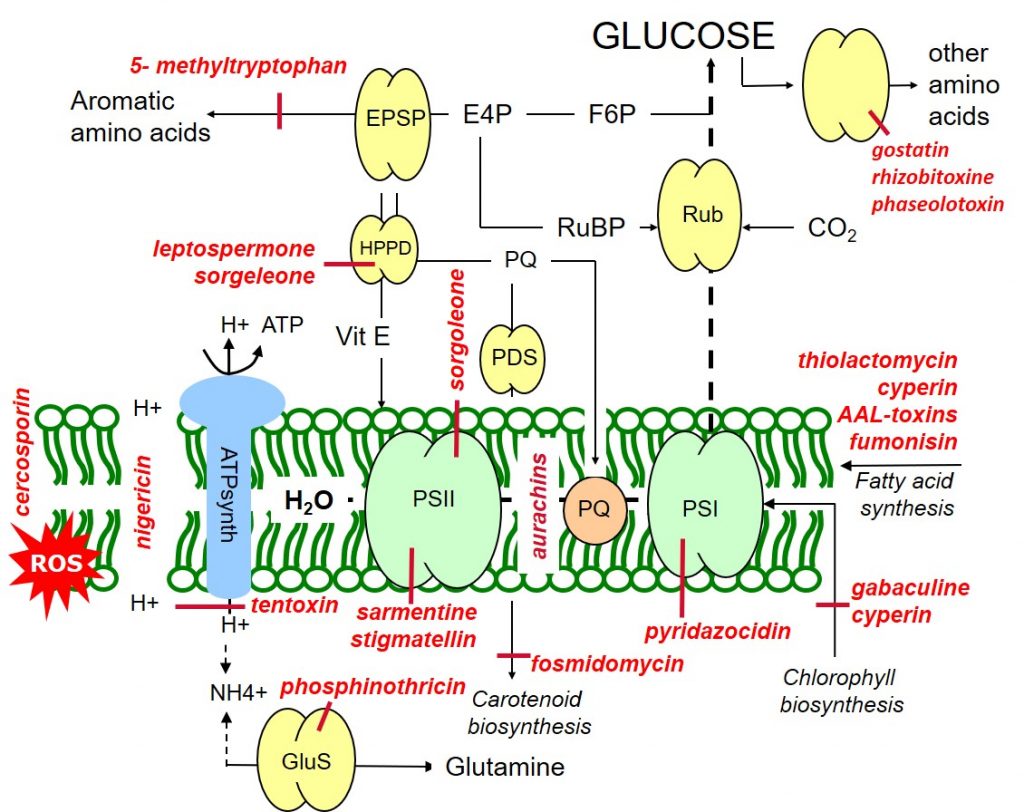
Figure 1: Target sites of natural phytotoxins affecting metabolic processes within the thylakoid membrane.
Gabaculine (Streptomyces toyacaenis) and cyperin (Preussia fleischhakii) are natural phytotoxins inhibiting chlorophyll biosynthesis. Carotenoid biosynthesis may be blocked at different sites by the action of fosmidomycin (Streptomyces lavendulae).
Leptospermone (Leptospermum scoparium) and sorgoleone inhibit the hydroxyphenylpyruvate dioxygenase (HPPD) enzyme, blocking both carotenoid biosynthesis and the formation of the antioxidant protectant, vitamin E. Like vitamin E, carotenoids have a protective antioxidant function through their quenching or scavenging of oxygen radicals.
Phytotoxins such as sarmentine (Piper longum) and cercosporin (Cercospora kikuchii) lead to loss of membrane integrity through reactive oxygen species (ROS), arising as a result of the over-reduction of the photosynthetic apparatus due to PSII inhibition.
The repair and maintenance of lipid membranes may be inhibited by herbicidal phytotoxins such as thiolactomycin (Streptomyces spp.) and cyperin (Preussia fleischhakii).
Biosynthesis of the aromatic amino acid tryptophan maybe inhibited by the phytotoxin 5- methyltryptophan (Cantharellus cibarius), while a range of phytotoxins such as gostatin (Streptomyces), rhizobitoxine (Bradyrhizobium) and phaseolotoxin (Pseudomonas) inhibit the biosynthesis of other amino acids. Phosphinothricin (Pseudomonas syringae) is a herbicidal phytotoxin inhibiting the synthesis of the amino acid glutamine.
Mitochondria-based metabolic processes inhibited by natural phytotoxins include respiration and lipid biosynthesis (Figure 2). Respiration can be blocked through the inhibition of NADH oxidation by fusicoccin (Fusicoccum amygdali). Syringomycin (Pseudomonas syringae) uncouples the proton gradient through the formation of membrane pores, while sorgoleone (Sorghum bicolor) and juglone (Juglans spp.) block proton-driven ATP synthesis via the H+ ATPase.
Fatty acid biosynthesis in the mitochondria can be inhibited by thiolactomycin (Streptomyces) and cyperin (Preussia fleischhakii).

Figure 2: Target sites of natural phytotoxins affecting metabolic processes within the mitochondria.
Thaxtomin (Streptomyces scabies) is a microbial phytotoxin that controls grass, broadleaf and sedge weeds by disrupting cellulose biosynthesis. Additional modes of action for bioherbicides include gene expression and regulation, as well as effects on hormonal processes such as jasmonate mimicking and auxin signalling.
BioInsecticide mode of action
Bioinsecticides include microorganisms that infect insects, and natural compounds with insecticidal activity sourced from microorganisms, plants or animals. Bioinsecticides known not to harm non-target (beneficial) insect species may be considered for use during flowering, a period where many conventional pesticides cannot be used.
Bioinsecticides fall into three major classes:
• Microbial bioinsecticides
• Plant-incorporated protectants
• Bio-derived (biochemical) bioinsecticides
Microbial insecticides include entomopathogenic fungi, capable of acting as pathogenic parasites of insects (and other invertebrates such as nematodes), and typically belong to the divisions Ascomycetes and Zygomycetes.
Besides their parasitic action, entomopathogenic fungi may also produce fungal toxins.
Entomopathogenic bacteria such as Bacillus thuringiensis (BT) are used to control insect pests, typically via their digestive tracts. Entomopathogenic viruses are specific to individual species: Cydia pomonella granulovirus specifically targets the codling moth C. pomonella (a major pest of fruit trees) killing the insect at the larval stage. Beneficial nematodes (e.g. Steinernema), while not microorganisms, are sometimes classified as such and are used in the biological control of insect pests.
Plant-incorporated protectants (PIPs) are a form of biological insecticide in which foreign DNA is inserted into crop genetic material (GM crops). Bacillus thuringiensis toxin genes may be directly incorporated into plants through the use of genetic engineering, giving these plants tolerance primarily to Lepidopteran insect pests, such as caterpillars and beetles.
RNA interference (RNAi) is an emerging technology, whereby RNA molecules capable of disrupting genes conferring tolerance to insecticides may be used to target resistant insects such as Potato Beetles.
Bio-derived (biochemical) insecticides are naturally occurring compounds, typically produced by plants for defense from predation, but also include microbial extracts that control insects or induce plant defense responses. Insect pheromones are used to attract insect pests to traps, or to disrupt mating cycles.
BT toxin crystals are microbial-derived insecticidal proteins which are activated upon ingestion by lepidopteran insect pests, leading to cell disintegration and insect death. Spinosad is a bioinsecticide based on natural metabolites, and is produced by fermentation of naturally occurring bacteria (Saccharpolyspora spinosa).
Surfactin, a biodegradable lipopeptide biosurfactant isolated from Bacillus species (see below) is a mosquitocide, Chitosan is a polysaccharide derived from fungi or crustacean shells, capable of inducing systemic resistance against insect pests.
Plant extracts such as garlic extracts or canola oil can repel insects, or act as antifeedants, while other plant extracts such as Nicotine (isolated from tobacco), Pyrethrins (isolated from Chrysanthemum plants) or Ryanoids (isolated from e.g. Ryania speciosa) have neural insecticidal activity, leading to hyperstimulation -or paralysis – and death (Figure 3).
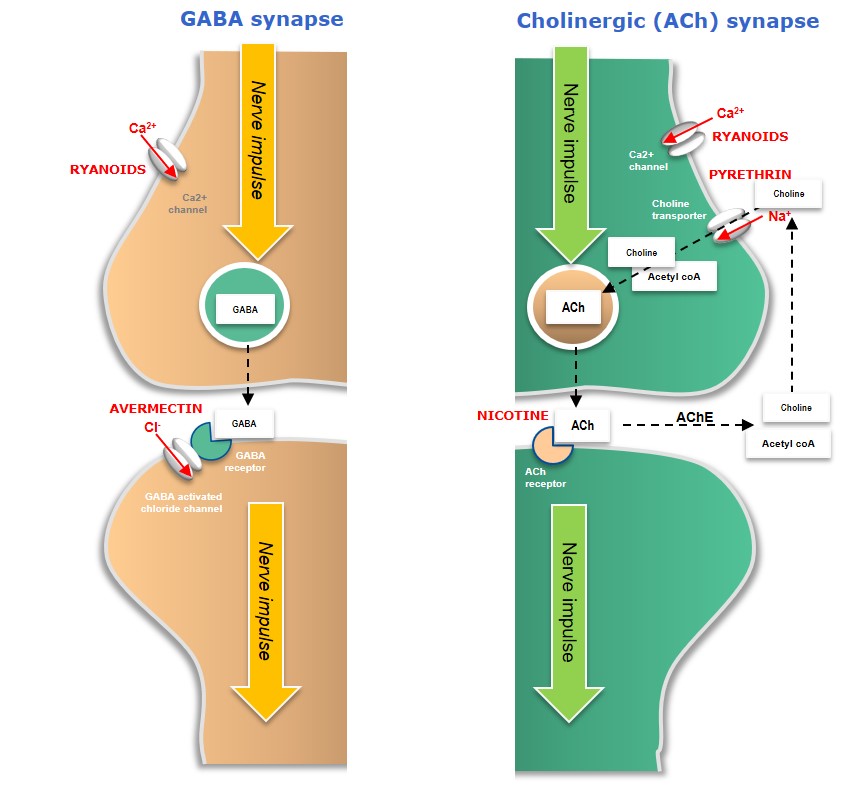
Figure 3: Mode-of-action of microbial and plant-derived neural bioinsecticides
In cholinergic (ACh) neurons, Nicotine competes with ACh neurotransmitters and competitively binds to the ACh receptor on the postsynaptic nerve, resulting in uncontrolled nerve stimulation (hyperstimulation) and death. Pyrethrins disrupt normal nerve function by binding to voltage-gated sodium channels, preventing them from closing normally and giving rise to neural hyperactivity and insect death.
Avermectins (naturally occurring compounds generated as fermentation products by the soil bacteria, Streptomyces avermitilis) affect insect-specific glutamate-gated chloride channels in GABA neurons, causing an influx of chloride ions (Cl-) into the postsynaptic neuron – leading to hyperpolarisation, paralysis and death.
Ryanoids are insecticidal alkaloids which interact with ryanodine calcium-channel receptors in insects, resulting in paralysis and death.
Other plant extracts such as Rotenone can inhibit mitochondrial electron transport (respiration). Finally, bio-derived insecticides include naturally occurring desiccants, such as borate or silica (diatomaceous earth, the fossilized remains of aquatic diatoms) which absorb the oil and fats from the cuticle of the insect’s exoskeleton, leading to desiccation and death.
BioFungicide mode of action
Biofungicides are formulations of living organisms or natural metabolites used to control the activity of plant-pathogenic fungi.
Many successful microbial biofungicides can colonize and grow in treated soil or on plant leaves, providing a long-term source of microbial and biochemical modes of action.
Biofungicide mode of action is complex and includes:
• antibiosis – microbial metabolites such as antibiotics or fungitoxins which inhibit spore germination or pathogen growth
• plant defense induction – the induction by biofungicides of localized or systemic defense mechanisms within the host plant
• exclusion – competition and displacement of pathogenic fungi from the rhizosphere or on the leaf surface by microbial biofungicides
• mycoparasitism – parasitization of pathogenic fungi by microbial biofungicides
• biostimulation – stimulation of plant growth and resilience
Antibiosis – The genus Bacillus comprises different strains which are commercially available as biofungicides. Bacillus strains such as B. subtilis, B. amylolquefaciens and B. licheniformis produce antimicrobial metabolites such as lipopeptides, which exhibit a broad antimicrobial spectrum and surfactant activity.
Due to their lipid-protein structure, lipopeptides can penetrate lipid membranes such as the cytoplasmic membrane of cells, affecting their integrity and ion permeability. The Iturin and Fengycin families of lipopeptides exhibit strong antifungal activity across a relatively broad range of pathogenic fungi, with fengycins having activity against Oomycetes such as Pythium and Phytophthora.
Another family of lipopeptides, Surfactins exhibit antiviral and antibacterial characteristics. Despite having low inherent antifungal activity, surfactins may act synergistically in combination with iturin.
Surfactins also function as biosurfactants, acting both as a wetter (by decreasing the surface tension of water droplets) and detergent, and are involved in biofilm formation and motility, while inhibiting the biofilm formation of phytopathogenic bacteria, interfering with their attachment to host plants.
Streptomyces is another naturally occurring gram-positive bacteria. It is found in soil, and can produce antimicrobial compounds (the antibiotic Streptomycin).
Induction of localized or systemic plant defense responses – Plants detect invading pathogens and induce defense responses, such as the production of toxic chemicals and cell destruction (hypersensitive responses), to hinder disease development. Plant defense responses are not initiated until pathogens are detected, due to the energy costs of their production and maintenance. Modern biopesticide strategies must thus consider the kinetics of defense response induction prior to pathogen attack.
These strategies include priming and activating plant defense responses prior to pathogenic attack, through application of microbial biofungicides, or through microbial metabolites such as Harpin, a natural protein found in common pathogenic microorganisms.
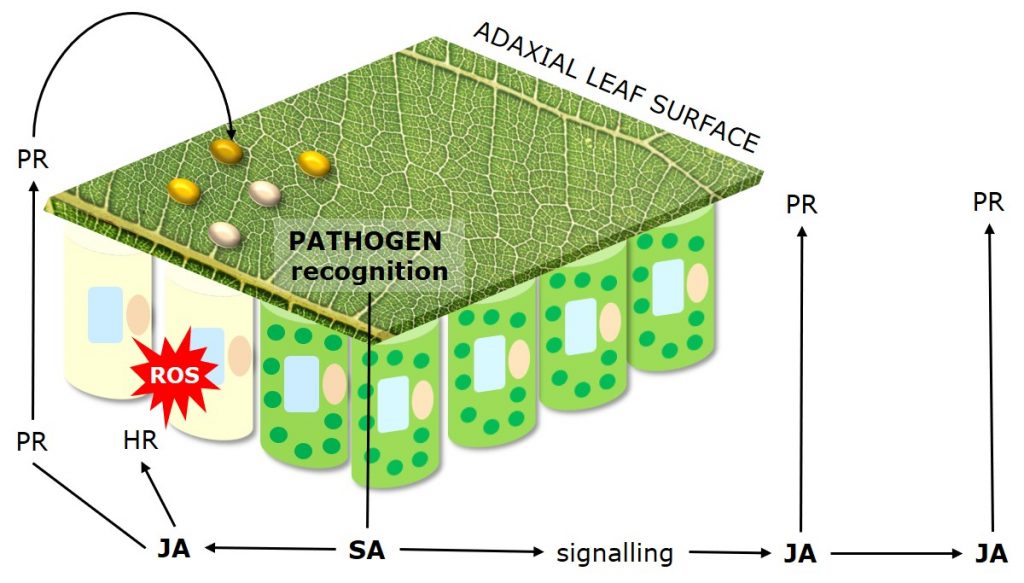
Figure 4: Mechanisms of Systemic Acquired Resistance (SAR) as plant response to pathogen attack.
Systemic Acquired Resistance (SAR) describes plant response to pathogen attack through priming of plant defense responses and compounds. Systemic Acquired Resistance is translocated within plants via the salicylic acid (SA) dependent signaling pathway.
Jasmonic acid (JA) mediates the systemic induction of disease defense compounds, including pathogenesis related proteins (PR-Proteins) such as chitinases and peroxidases which degrade fungal cell walls, and antifungal phytochemicals such as phenolics, terpenoids and alkaloids.
Besides direct antifungal strategies, plants may activate a hypersensitive response (HR), also called “programmed cell death,” which limits the access of biotrophs that feed on living tissue to water and nutrients.
The hypersensitive response is a mechanism used by plants to restrict pathogen development by inducing the localized, rapid death of cells in the region immediately surrounding an infection.
Following recognition of the pathogen the activation of resistance genes triggers an oxidative burst of reactive oxygen species (ROS). Reactive oxygen species draw electrons from chloroplast thylakoid membranes (lipid peroxidation), leading to the loss of chlorophyll and associated chlorotic lesions.

Figure 5: Hypersensitive response with associated chlorotic lesions in soya leaves following inoculation with soya rust.
Exclusion – Microbial biofungicides may compete with plant pathogens for nutrients and infection sites on leaf surfaces and in the soil. Some microbial biofungicides metabolize root exudates that stimulate pathogen germination, reducing their ability to infest plants.
Microbial biofungicides such as Trichoderma (a genus of soil fungi) may attack and suppress plant pathogens through a combination of displacement and the production of antimicrobial compounds such as cell-wall degrading enzymes.
Plant Growth Promotion – Biostimulation and protection against biotic (weeds, pests and diseases) and abiotic stresses have been reported for many agricultural biologicals, including biopesticides, by improving plant growth and root development (improving drought resistance and nutrient uptake). Increased crop vigor ensures optimal natural crop defense responses.
Biotic and abiotic plant response mechanisms include photoreceptors, proximity sensors and accelerometers, and may be compared to smartphone sensors. Plants mediate defense strategies through complex communications systems, which may be compared to 3G (volatile plant hormones) and Wi-Fi (root-root and root-mycorrhiza interactions).
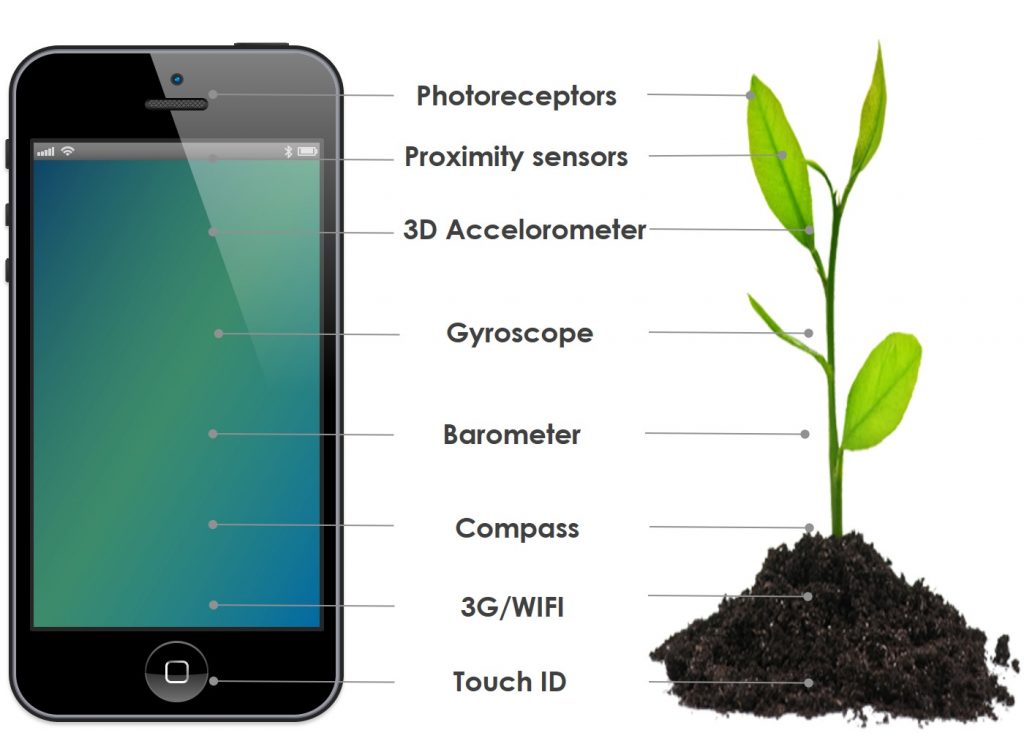
Figure 6: Comparison of plant environmental, pathogen and pest response mechanisms to smartphone sensors.
Since many biopesticides are slow to control the pest or pathogen, growers may feel that the product has insufficient effect and reapply unnecessarily. Diligent reading of labels and understanding how the product works – their mode of action – is necessary for the optimal application of these products, allowing growers to reap the full benefits and attain consistent results.
Attention is also being given to delivery and application practices, specifically to understanding the impact of plant defense induction kinetics on application timing and placement.
Because the mode of action of a biopesticide typically differs from that of a conventional pesticide, the efficacy of the biopesticide must also be assessed differently – not only through field trials but also by estimating growth, stress tolerance, yield and quality of the final product. It is crucial to understand how a biopesticide works in a cultivation program, and to thoroughly test the product based on all potential benefits.
Efficacy trials demonstrate conclusively that biology-based crop protection strategies can attain an efficiency at least on par with conventional crop protection strategies, when applied with due regard for factors such as plant-response kinetics and disease pressure development.
Reports of inconsistent efficacy lead to either over-optimism or skepticism by corporate decision makers and farmers alike. From a grower / end-user point of view, an important strategic strength of biopesticides lies in their application at specific timings: short preharvest intervals allow the application of biopesticides (especially biofungicides) immediately before harvest, where few (if any) conventional pesticides are available. Crop protection biologists have a unique opportunity to mediate an understanding of biopesticide mode of action, and to ensure the implementation of this knowledge in the development of commercial strategies.
Thanks for reading – if you liked what you read, please consider liking and sharing. Your feedback is invaluable, and helps new readers discover my work!
Please feel free to read and share my other articles in this series:
A little about myself
I am a Plant Scientist with a background in Molecular Plant Biology and Crop Protection.
20 years ago, I worked at Copenhagen University and the University of Adelaide on plant responses to biotic and abiotic stress in crops.
At that time, biology-based crop protection strategies had not taken off commercially, so I transitioned to conventional (chemical) crop protection R&D at Cheminova, later FMC.
During this period, public opinion as well as increasing regulatory requirements gradually closed the door of opportunity for conventional crop protection strategies, while the biological crop protection technology I had contributed to earlier began to reach commercial viability.
From January 2018, I will be available to provide independent Strategic R&D Management as well as Scientific Development and Regulatory support to AgChem & BioScience organizations developing science-based products.
For more information, visit BIOSCIENCE SOLUTIONS – Strategic R&D Management Consultancy
Keywords: #cropprotection #biologicals #biopesticides #pesticides #formulation #plantscience #agchem #agbio #bioscience #authorpreneur #projectmanagement #biological #biopesticide #pesticide #R&D #strategy #consultant #strategic #management #executive #insead #srdm
Harry Teicher is the founder of BIOSCIENCE SOLUTIONS and an Authorpreneur, providing organizations with Strategic- and Project Management as well as Development & Communication solutions. He is an Administrator of the INSEAD Strategic R&D Management Alumni Network LinkedIn group. Follow him on Linkedin, Twitter and Facebook.