STRATEGIC R&D MANAGEMENT PART II: Succesful Biology-based Product Development – a Case Study
Strategic R&D Management case study presented by Harry Teicher at “R&D of AgriProducts: Exploring the next generation of AgriProducts”, Amsterdam 22-23 November, 2017.
The objective of this case study is to address issues commonly encountered at the interface between strategic management and expert teams involved in conventional and biological crop protection development.
In the first part of this series on Strategic R&D Management, issues encountered through the implementation of a conventional development strategy for the development of a new biological product were identified.
In the second part of this series, the learning process is applied to develop a new biological development strategy leading to the successful introduction of a new product line.
• Case study of experiences developing a new biological product – what did we learn?
• To be successful, Strategic Management need to understand the biology involved – example of when chemists tried working with biologicals and things did not go to plan
• Why it is key that senior management from chemical companies and chemical backgrounds need to be well informed when it comes to biologicals if they are looking to developed integrated products
Case study: Biology-based Product Development in the Agrochemical Industry
DISCLAIMER: The following case study is fictitious and based on experiences and observations made across the crop protection industry, and does not represent the experiences of any specific company.
The case study began with the initial strategy for the development of a new biological product by AGCHEMCO, a conventional (chemical) crop protection company. It identified issues encountered through the implementation of a conventional development strategy.
Summary of PART I:
In May 2014 Mary Walker, CEO of AGCHEMCO (an agrochemical company specializing in conventional crop protection), presented an ambitious strategy: (i) the immediate implementation of drastic cost-cutting measures and the culling of low-profit product lines to create a leaner, more effective core business, and (ii) entry into the rapidly growing biopesticide market.
Jon Braun, VP of R&D for AGCHEMCO was assigned the responsibility for the development of AGCHEMCO’s first viable, competitive biological product.
Development was to be based on AGCHEMCO’s existing development protocols, with the objective of developing a product capable of working at least as effectively – and as consistently – in the field as the existing agrochemical competitors.
The initial development results were disappointing, and exposed the one weakness that gave AGCHEMCOS Executive Management greatest cause for concern: the variable efficacy of biological crop protection products under field conditions.
With the decline in their core business halted, AGCHEMCO debated dropping their foray into biological crop protection in order to focus resources on the optimization of their conventional agrochemical portfolio.
Lessons learnt
An initial finding was that existing biological knowledge had not sufficiently been incorporated into the product development strategy. This led to inappropriately-designed lab and field efficacy studies (Figure 1).
By focusing on competitiveness against conventional products and using standard protocols for conventional agrochemical product development, trials had been set up as protective and curative essays, with application timings 24 hours before inoculation for protective assays, and 24 hours after inoculation for curative assays. The efficacy of the biofungicide was compared to commercial agrochemical references.
This timing did not allow for the extended kinetics of plant defence introduction necessary for for optimal biopesticide efficacy, leading to poor efficacy results compared to the commercial references. The kinetics of plant defence response lead to the biopesticide performing adequately in petri-dish but not in whole-plant essays.
Furthermore, this lead to excessive variability in field trial efficacy data: trials in regions exposed to disease pressure immediately after the application of the product showed low levels of biofungicidal efficacy (there was no time for the induction of plant defence responses), while trials carried out in regions where inoculum appeared later showed greater levels of efficacy.

Figure 1: Issues in the development of a new biological product using a incremental R&D framework.
There was initially little incentive or opportunity to challenge the development process by integrating biological learning into the development strategy, as the performance of the project was measured on output based KPI’s (Figure 1). In addition, the stage gate nature of the project did not provide resources or flexibility for iterative development ie. the implementation of experimental learning into the redesign of new trials.
A stage gate process is part of a project management framework in which a product is divided into distinct stages separated by decision points known as gates. While stage gate processors may be appropriate for conventional incremental project management, the iterative process of innovation can be hindered, and complex innovation projects may be halted importunely.
By strictly adhering to a stage gate process, the inability to significantly develop an innovation product could lead to the rejection of the project on the basis of its inability to deliver a viable competitive product.
For innovation projects, an iterative gate process can be more appropriate. In our example, iteration and the integration of biology into strategy would allow focus on optimisation of the application of the product, rather than optimisation of the product itself.

Figure 2: Learnings – the development of a new biological product using an iterative R&D framework.
To understand the value of integrating biological (“bottom-up”) input into the strategic development process, we need to return for a moment to the original SWOT analysis, and to evaluate it from the point of view of a biologist, as opposed to the conventional of view of a chemical engineer.
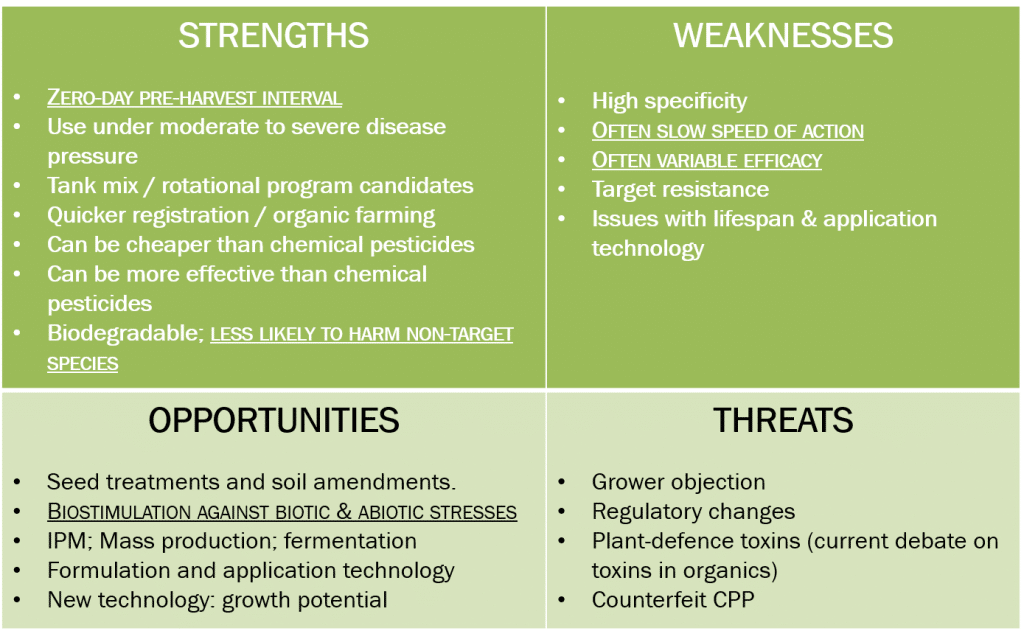
Figure 3: SWOT analysis, BioPesticides (AGCHEMCO).
From a biological / end-user point of view it is clear that an important strategic strength of biopesticides lies in their application at very specific timings: short preharvest intervals allow the application of biopesticides (especially biofungicides) immediately before harvest, where very few (if any) conventional pesticides are available.
Biopesticides (especially bioinsecticides) known not to harm non-target (beneficial) insect species may be applied during flowering, a period where many conventional pesticides are not able to be used due to their effect on bees. Accordingly, if the biopesticide candidate is to be strategically placed to utilise these windows of opportunity, there is not much point in comparing their efficacy with commercial references which are not relevant.
Biostimulation and protection against biotic and abiotic stresses have been reported for many agricultural biologicals. Inclusion of biostimulation and stress alleviation evaluations in the design of biopesticide efficiency trials provides important decision-making parameters for the strategic viability of the tested product.
Often, the only biological parameter considered when evaluating the product is its speed of action and efficacy. However, an understanding of the kinetics of plant defence induction will allow the design of appropriate trials, and the inclusion of appropriate measures of comparison.
Accordingly, for our example, curative experiments might have been eliminated, as many biopesticides attain their effect through the induction of plant defence responses. As these responses must be induced in advance of the pathogen reaching the leaf surface, protective applications are considered most appropriate for these products.
Rather than performing curative trials, resources could be allocated to optimising the induction of plant defence responses, for example by formulating the biofungicide in yeast cells (natural microcapsules), which contribute to the induction of defence responses (the yeast cells are recognized as possible pathogens) while protecting the biopesticide from desiccation and UV degradation.
The development of natural plant-defence mechanisms over at least 100M years has lead to a diverse global flora. It is this diversity of wild flora which ensures natural disease pressures remain at a controllable level. In monocultures, disease pressures can be exponentially higher, and the timely application of biopesticides in conjunction with conventional pesticides is vital to control disease pressures and ensure optimal biopesticide efficacy.
Mediating biology-based strategies to decision-makers
From the above, it can be seen that one of the key issues in the mediation of biology-based strategies to conventional (chemical)-based decision-makers lies in conveying the nature and complexity of biotic and abiotic factors in the timing of these mechanisms and their induction.
Too often we experience the frustration of inconsistent efficacy following the application of biologicals when disease pressures are already too high. When applied with regard for factors such as plant-response kinetics and disease pressure development, these same products prove to be highly efficacious.
Currently, reports of inconsistent efficacy lead to either over-optimism or skepticism on the part of corporate decision makers and farmers alike. Crop protection biologists have a unique opportunity to mediate an understanding of the drivers of biologicals efficacy, and to ensure their implementation in the development of commercial strategies.
For many conventional companies, this can be achieved through the implementation of Inclusive Strategic R&D Management frameworks.
Inclusive Strategic R&D Management Frameworks.
FAIR PROCESS: facilitating inclusion of Execution (bottom-up) into Strategy (top-down)
In order to ensure the inclusion of expert biological knowledge into R&D and business strategies, it is necessary to evaluate the companies strategic framework and to consider implementing some degree of change management.
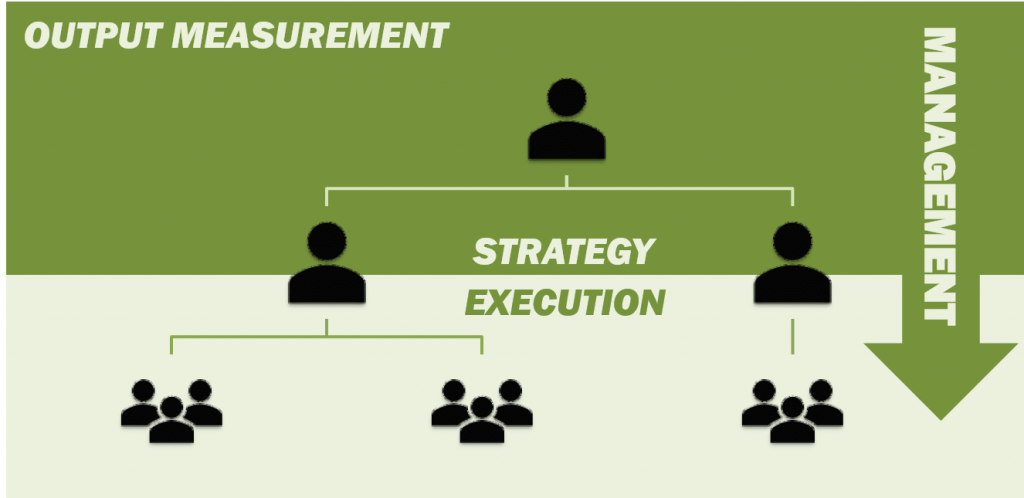
Figure 4: Top-down management framework.
Traditionally, crop protection companies have favoured incremental, product-orientated development. Management levels have been responsible for defining the company’s vision and strategy and for communicating this strategy in a “top-down” or “management-down” framework (figure 4). In this framework, execution of the strategy is carried out by the “DOWN” or EXPERT level. This level typically has the greatest customer or end-user contact, and receives important information necessary for product development.
Conventional OUTPUT-based project management tools and evaluations (e.g. KPI) are considered appropriate for incremental, product-orientated development frameworks, and are especially relevant for the “TOP” or MANAGEMENT team (who after all are responsible for product output).
When companies disrupt their core business and introduce innovation projects, it may become necessary to revise this development framework.
An INPUT-based, iterative project management system and INPUT-based evaluation can be introduced for INNOVATION projects, in order to maximise the LEARNING process while motivating the “DOWN” or “EXPERT” team.
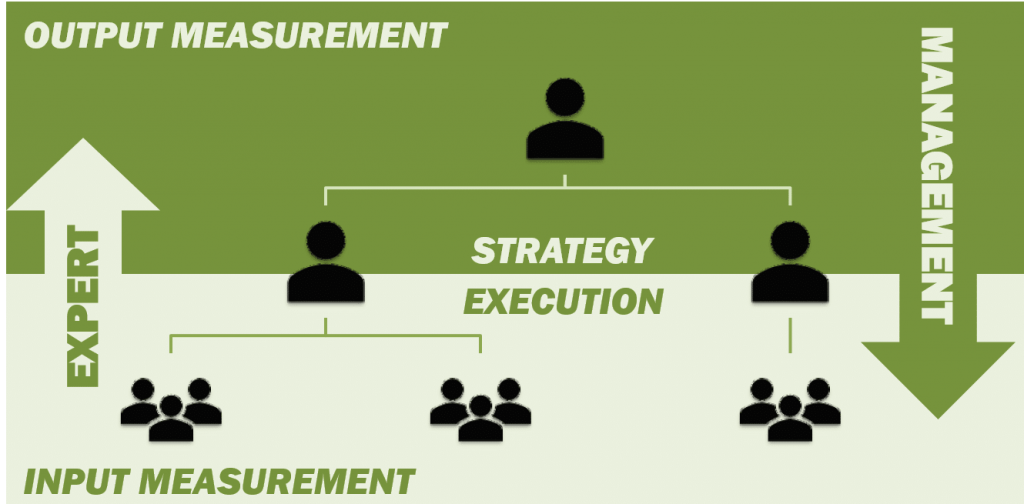
Figure 5: Fair process management framework.
An example of an iterative, input-based development framework is FAIR PROCESS MANAGEMENT (FPM) – a process developed by INSEAD (Blue Ocean Strategy) to facilitate inclusion of Execution (bottom-up) into Strategy (top-down) by creating individual buy-in at the beginning of the process.
The objective of FPM is to optimize strategic decisions by management (the “UP” team) as well as commitment from those involved in execution (the “DOWN” team).
To facilitate the implementation of an INPUT-based, iterative development framework (figure 5), it is necessary to ensure open communication at the critical interface between strategy (vision) and execution.
For the development of biology based products, this requires the “buy-in” and inclusion of biology experts for strategic decisions, to the same level as chemists and chemical engineers who drive strategic decisions for conventional products.
The objective is to provide an inclusive, iterative development framework to promote independent innovation initiatives and autonomous strategic biological input – “Frame Strong, Manage Light”.
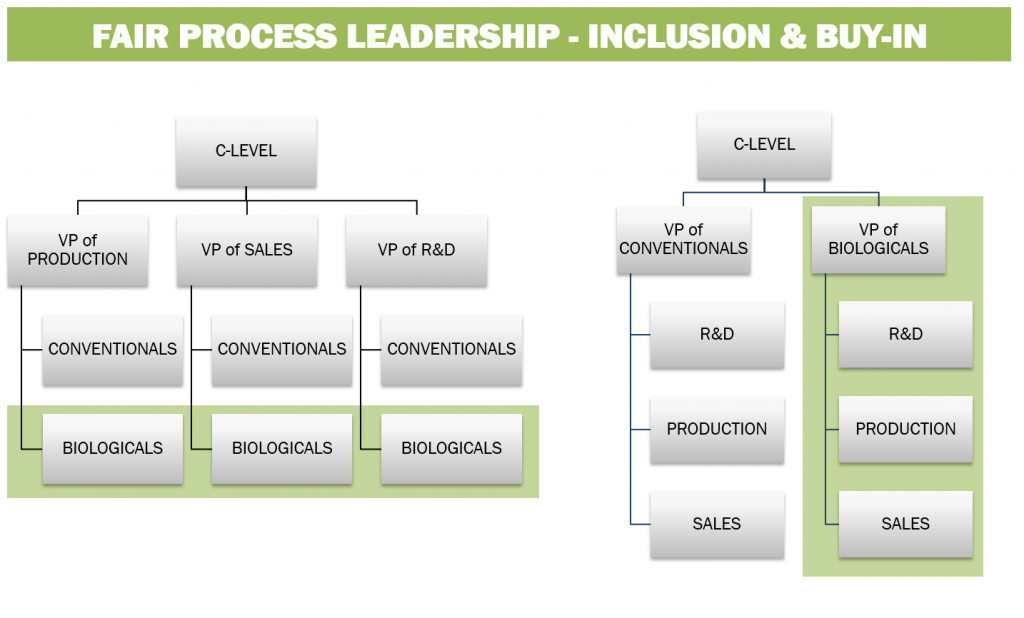
Figure 5: Organisational frameworks for the integration of biologicals into conventional crop protection organisations.
To achieve this, biological development groups should not merely function as support functions, but should be elevated to an increased strategic responsibility within conventional crop protection organizations (Figure 5, left).
For companies investing heavily in biologicals development, it may be necessary to radically restructure the organisation of the company (Figure 5, right) in order to adapt to the requirements for both incremental and iterative development frameworks.
A radical reorganisation makes even more sense when we consider the economical and temporal differences between conventional and biological crop protection development.
In the figure below, it can be seen that for conventional chemistries, development times can be three times longer then for biological crop protection development, while expenses may be as much as 10 times greater.
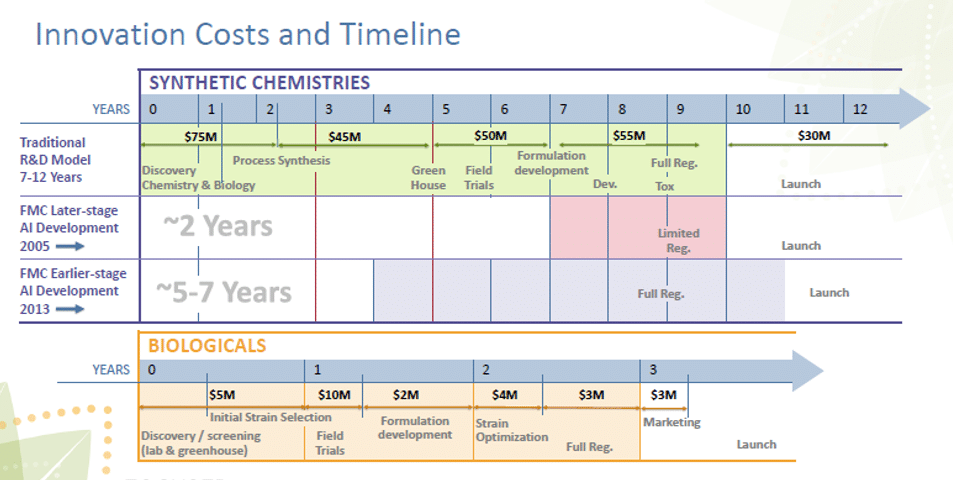
Figure 6: Innovation costs and timelines for conventional and biological crop protection development (from: FMC – Investor Relations Solutions, FMC, 2015).
FAIR PROCESS LEADERSHIP & TEAM MANAGEMENT
To successfully manage the CRITICAL INTERFACE BETWEEN STRATEGY (VISION) & EXECUTION for innovation portfolios, it is necessary to introduce an iterative, input-based development framework to promote the inclusion and buy-in of expert teams. The following questions are those typically addressed in this process:
(I) How do we ensure a speak-up culture?
Traditionally, crop protection companies have implemented a classical management career track. In this system, competent R&D scientists are promoted to management positions, and it is from these positions that contributions are made to business strategy.
Unfortunately, it is not always the case that the most competent experts become the best managers or strategists, and it is quite common for excellent scientists to attain management positions and subsequently leave the company to return to the bench. Alternatively, talented scientists may refuse promotion to managerial positions, and their input to strategic decisions is lost.
The introduction of expert-track career pathway is a way to provide experienced and talented scientists with the opportunity to contribute to business strategy at a managerial level, without the constraints of a managerial career track.
Here, talented scientists progress through Senior- and Principal Scientist levels to the position of Research Fellow, at which level they have the same opportunity to provide strategic input as e.g. an R&D Director.
(II) How do we promote bottom-up initiatives?
Conventional, output-based development frameworks are appropriate for the management of incremental (conventional) development projects, and output-based measures of performance such as KPIs are easy to define.
However, incremental frameworks may hinder the iterative R&D process of innovation, and KPI’s and bonus incentives can prove detrimental to the innovation process. Simply put, bonuses do not always motivate scientists.
For input-based projects, one objective of measuring performance is to provide a means to END the innovation process (e.g. by defining a MINIMUM VIABLE PRODUCT).
For innovation projects, MEASURES OF INPUT-BASED PERFORMANCE can be more appropriate, although the identification and quantification of these performance measures proves challenging for many companies. An example of an appropriate measure could be the quantification and recognition of learning provided to the project or company by the individual.
(III) How do we lead and motivate expert teams?
Innovation and inclusion of expertise from the EXECUTION or DOWN layer entails recognising and legitimising innovation and learning inputs as a measure of input-based performance. At the same time, clear expectations of independent, individual innovation should be included in R&D job descriptions.
R&D experts should always have the freedom to challenge the existing R&D framework, to ensure “bottom-up” integration, for example by critically evaluating project-group makeup – “is Biology adequately represented throughout the project timeplan?” and “do evaluation criteria also measure learning?”.
Rather than monetary bonuses, R&D staff may be better motivated through e.g. the provision of time for them to pursue non-project related innovation initiatives, opportunities for scientific publication and increased access to scientific congresses and conferences not necessarily relevant for the company’s core business.
Increased flexibility in working hours and locations for R&D staff as well as increased autonomy have also proven to be effective motivators.
Finally, the visible inclusion of R&D input into strategic business decisions by encouraging a speak-up culture and promoting bottom-up initiatives is a key factor in ensuring the motivation of expert teams.
***
Thanks for reading – please help make LinkedIn great and enable me to produce more content by LIKING or SHARING this article, making it visible to your network!
***
A little about myself
I am a plant scientist with a background in Molecular Plant Biology and Crop Protection. 20 years ago, I worked at Copenhagen University on photosynthetic responses to stress in crops. Subsequently, I worked in Australia on molecular defence mechanisms induced by Phylloxera attack in grapevine.
At that time, biology-based crop protection strategies had not taken off commercially, so I transitioned to conventional (chemical) crop protection R&D at Cheminova, later FMC.
During this period, public opinion as well as increasing regulatory requirements gradually closed the door of opportunity for conventional crop protection strategies, while the biological crop protection technology I had contributed to earlier began to reach commercial viability.
From January 2018, I will consolidate 17+ years of industry experience in BioScience R&D management with my academic research background, to provide independent Strategic R&D Management as well as Scientific Development and Regulatory support to AgChem & BioScience organizations developing science-based products. For more information, visit BIOSCIENCE SOLUTIONS – Strategic R&D Management Consultancy
***
Harry Teicher is the founder of BIOSCIENCE SOLUTIONS and an Authorpreneur, providing organizations with Strategic- and Project Management as well as Development & Communication solutions. He is the Administrator of the INSEAD Strategic R&D Management Alumni Network LinkedIn group. Follow him on Linkedin, Twitter and Facebook.



