The Labcoat Guide to Pesticide Mode of Action: Herbicides – Part I
In this first article in the Labcoat Guide to Pesticide Mode of Action, I focus on conventional herbicides and discuss their Biochemical Mode of Activity and associated plant symptoms.
A little about my background
I am a plant scientist with a background in Molecular Plant Biology and Crop Protection. 20 years ago, I worked at Copenhagen University on photosynthetic responses to stress in crops. Subsequently, I worked in Australia on molecular defense mechanisms induced by Phylloxera attack in grapevine.
At that time, biology-based crop protection strategies had not taken off commercially, so I transitioned to conventional (chemical) crop protection R&D at Cheminova, later FMC.
During this period, public opinion, as well as increasing regulatory requirements, gradually closed the door of opportunity for conventional crop protection strategies, while the biological crop protection technology I had contributed to earlier began to reach commercial viability.
From January 2018, I will consolidate 17+ years of industry experience in BioScience R&D management with my academic research background, to provide independent Strategic R&D Management as well as Scientific Development and Regulatory support to AgChem & BioScience organizations developing science-based products.
For more information, visit BIOSCIENCE SOLUTIONS – Strategic R&D Management Consultancy.
Herbicides – also known as weedkillers – are substances toxic to plants that are used to destroy unwanted vegetation (weeds).
Herbicides control weeds by interfering with their growth. This is achieved through a number of different Modes of Action (MOA).
MOA may be differentiated into Mechanism of Action, referring to the biochemical site of herbicide activity, and Mode of Action – the associated plant symptoms that occur following herbicide application. In the following, Mode of Action will be used in the following to describe both the site of activity as well as associated plant symptoms.
A sound knowledge of herbicide mode of action is important for determining how to optimally use a herbicide, as well as for understanding the underlying mechanisms of weed resistance and herbicide selectivity.
In herbicide R&D, elucidating herbicide mode of action requires years of research, coupling biochemical and molecular biological knowledge with plant physiology and the timing of symptom development.
Herbicides and enzyme function
Generally, herbicides target specific enzymes and inhibit their normal function. By inhibiting enzyme function, herbicides prevent the catalysis of the biosynthesis of organic plant constituents, for example fatty acids, which are necessary for plant function.
Fatty acids are an integral component of plant cell membranes and are vital for plant growth and development.
Their synthesis implants is catalysed by enzymes. In the following figure, the enzyme (in green) functions normally, catalysing the conversion of precursors to fatty acids.

Figure 1: Fatty acid biosynthesis.
The herbicide Fenoxaprop inhibits the enzymatic process of fatty acid biosynthesis (Figure 2) by specifically binding to the ACCase (acetyl-CoA carboxylase) enzyme. This binding is dependent on compatibility between the three-dimensional binding site on the enzyme, and the three-dimensional shape of the herbicide molecule.
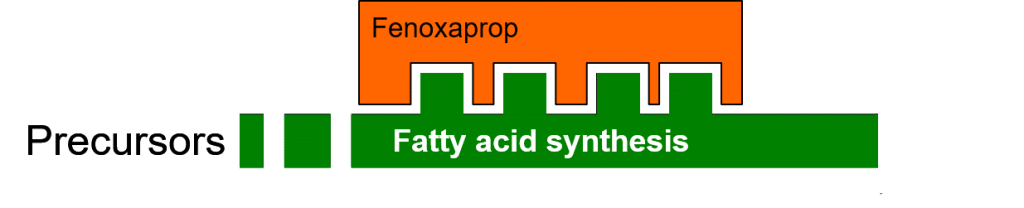
Figure 2: Herbicidal inhibition of fatty acid biosynthesis.
Affected plants are unable to synthesise new cell and organelle membranes, or maintain and repair existing membranes damaged by reactive oxygen species. Herbicides may thus restrict or stop plant growth, give rise to an accumulation of toxic precursor molecules, or they may lead to a decreased scavenging of reactive (radical) oxygen species.
Herbicide selectivity, resistance and safening.
Non-selective herbicides kill all plant material with which they come in contact, while selective herbicides kill weed targets, leaving the crop relatively unharmed. Herbicides may be selective to plant species possessing a specific enzyme, while weeds may mutate their enzymes and establish a resistant population.
Plants may rapidly metabolise herbicides, or may possess mechanisms to alter the shape and enzyme–binding compatibility of herbicide molecules e.g. by conjugating the herbicide molecule with a glutathione molecule. This is the basis of herbicide safening, to be discussed in a later article.
Herbicide classification
Herbicides are classified according to their site, or mode of action. The primary classification based on sites of action includes:
- Photosystem II inhibitors
- Photosystem I inhibitors
- EPSPS inhibitor
- HPPD inhibitors
- PDS inhibitors
- ACCase inhibitors
- ALS inhibitors
- Glutamine synthetase inhibitors
- Synthetic auxins
- Cell Division inhibitors
Herbicides and photosynthesis
Most commonly used herbicides affect photosynthesis, either through a direct inhibition of photosynthetic electron transport, or through the creation of reactive oxygen species with resultant photooxidative damage, or by inhibiting protective mechanisms capable of quenching reactive oxygen species.
Photosynthesis is a process which occurs primarily in plants and some bacteria. This suggests that herbicides specifically target plants, as opposed to beneficial mammals and (most) microorganisms.
From a toxicological point of view it is important to understand that mitochondrial (respiratory) electron transport is evolutionarily related to photosynthetic electron transport, and that some herbicides may affect respiration in mammals, insects and microorganisms. This will be discussed in a subsequent article.
Photosynthesis is the process where plants use light energy to convert CO2 and H2O to sugars, with the concomitant release of oxygen.
Photosynthesis primarily takes place in the leaves of green plants. Within plant cells, chloroplasts contain the enzymes necessary for photosynthesis, bound within stacked membrane systems called thylakoids.
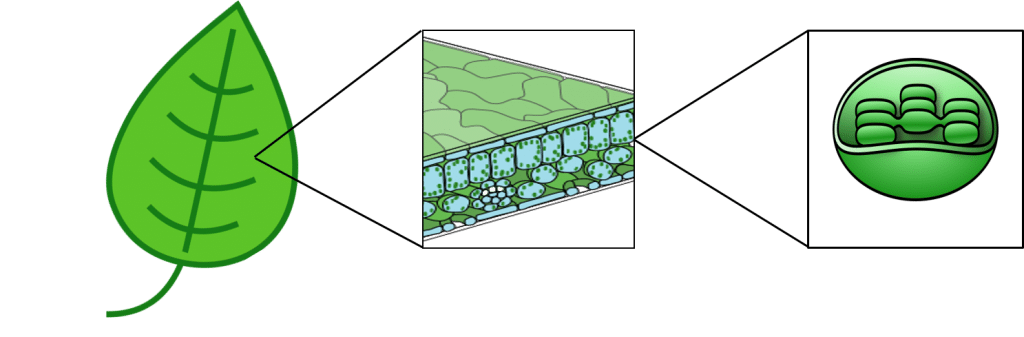
Figure 3: The location of chloroplasts within plant leafs.
Thylakoid membranes provide a lipophilic support structure for photosynthetic enzyme complexes (Photosystem One; PSI and Photosystem Two, PSII) as well as for mediators of electron transport such as plastoquinone, PQ.
Photosynthetic electron transport and reactive oxygen species
In figure 4, light can be seen to mediate the photolysis of water to oxygen (O), protons (hydrogen ions, H+) and electrons, and drive these electrons against a redox gradient through PSII and PSI, permitting the reduction of CO2 to glucose.
Plastoquinone mediates the transfer of electrons from PSII to PSI, and contributes to maintaining the redox balance between both photosystems.
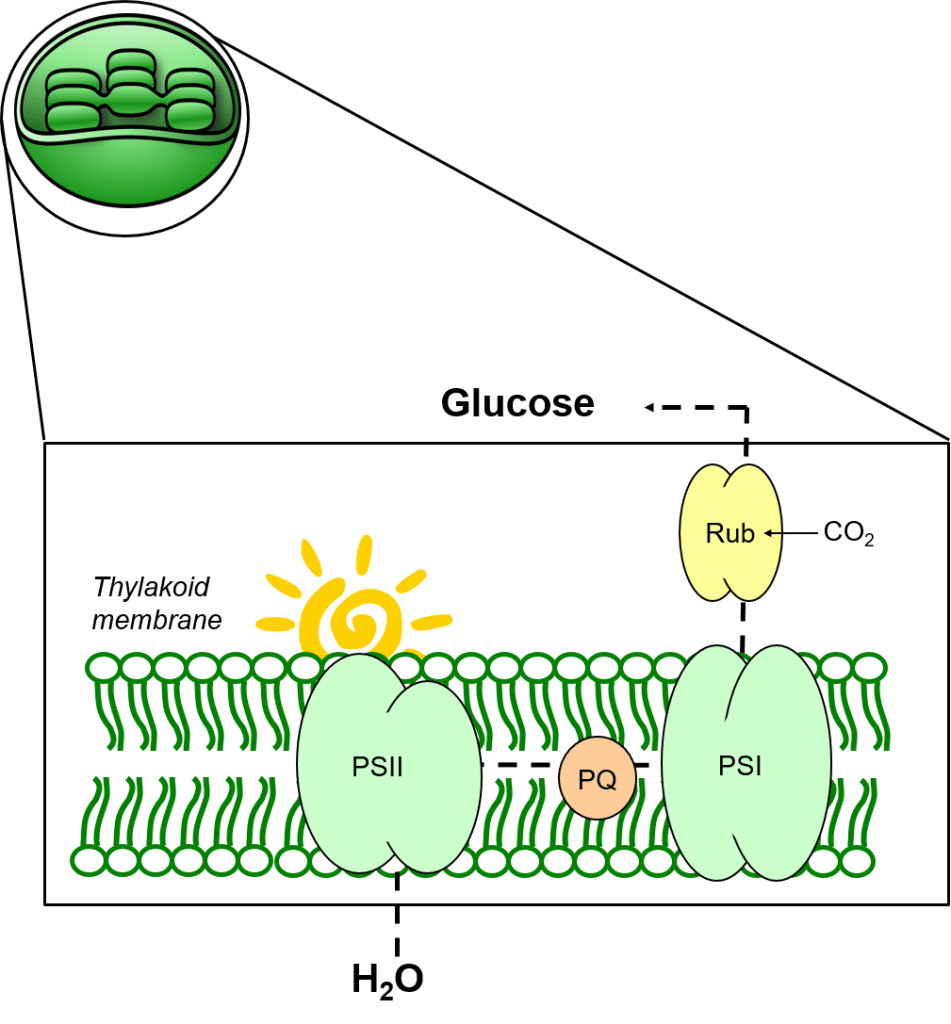
Figure 4: The process of photosynthesis in thylakoid membranes.
Herbicidal photosynthesis inhibitors
Herbicides of the PSII- and PSI-inhibitor group prevent the normal functioning of electron transport in the photosynthetic apparatus (Figure 5). This leads to the over-reduction of photosynthetic components and the reaction of excited state electrons with oxygen molecules, leading to the creation of reactive oxygen species (ROS).
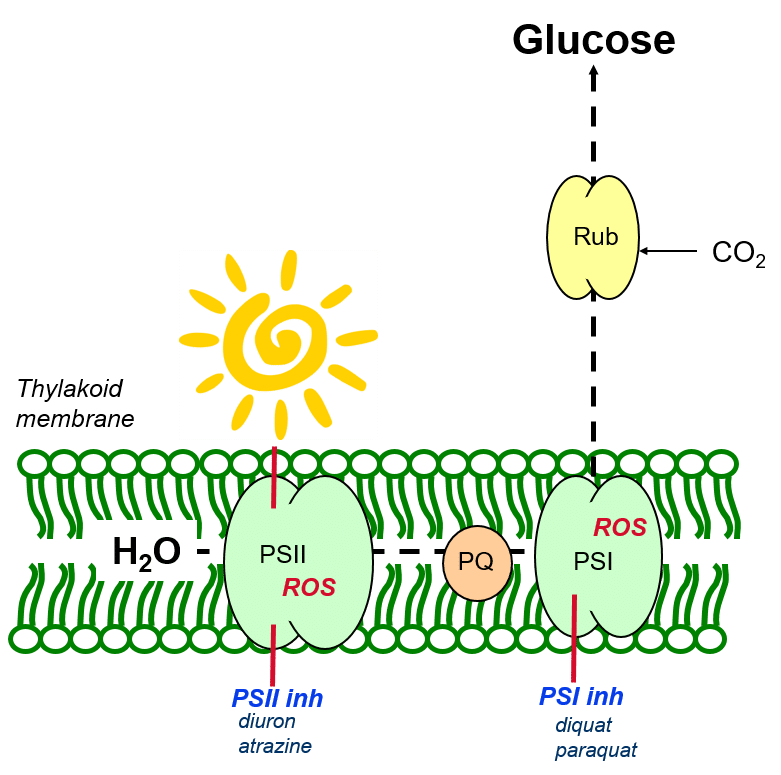
Figure 5: The herbicidal activity of PSII- and PSI-inhibiting herbicides.
Reactive oxygen species (or oxygen radicals) attempt to stabilise their electron states by drawing electrons from surrounding thylakoid membrane, in a destructive process termed membrane peroxidation.
Membrane peroxidation leads to the oxidative destruction of membrane fatty acids – the thylakoid membranes lose their integrity and are no longer able to hold the green chlorophyll molecules which give the leaves their characteristic colour. Accordingly, affected leaves may show typical symptoms of oxidative damage (necrosis, or burning) or bleaching associated with chlorophyll loss.
Inhibition of aromatic amino acid biosynthesis
Aromatic amino acids are created from carbon building blocks derived from glucose (figure 6) in a process catalysed by the enzyme EPSP (5-endopyruvylshikimate-3 phosphate synthase) which couples an aromatic carbon ring to precursor molecules (building blocks). The herbicide glyphosate inhibits the catalytic function of EPSP, thereby inhibiting the formation of aromatic amino acids necessary for plant growth and development.
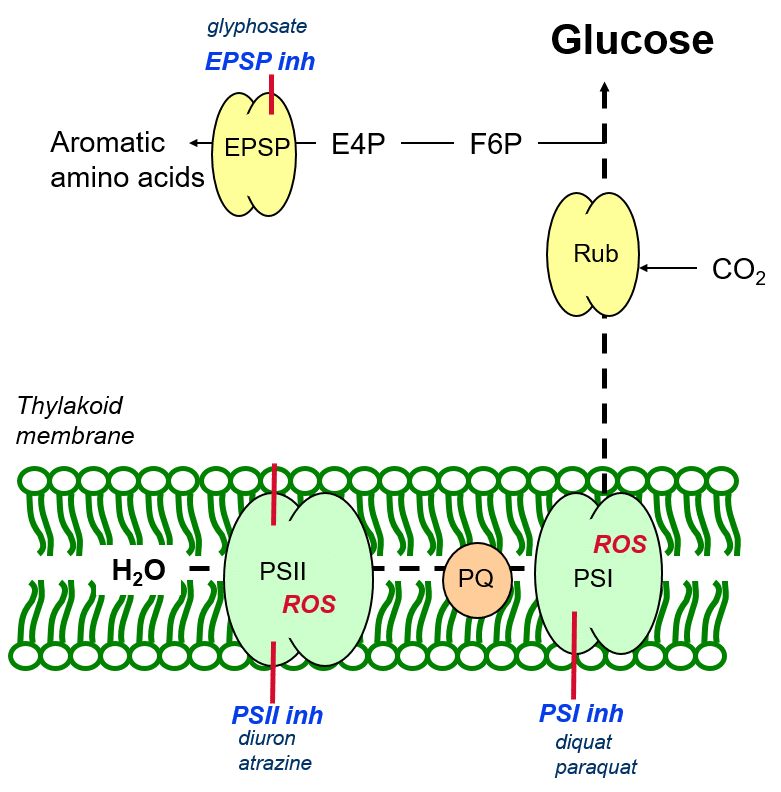
Figure 6: Inhibition of aromatic amino acid biosynthesis
HPPD and PDS inhibitors
In addition to aromatic amino acid biosynthesis, EPSP catalyses the formation of other aromatic molecules such as the protective antioxidant, Vitamin E (Figure 7) – an oxygen radical quencher, and plastoquinone, a mediator of photosynthetic electron transport as well as a precursor in carotenoid biosynthesis.
Vitamin E synthesis is catalysed by the HPPD (hydroxyphenylpyruvate dioxygenase) enzyme, using aromatic precursors synthesised by the EPSP enzyme. A number of herbicides inhibit the enzymatic activity of HPPD, and these are termed HPPD-inhibitors. By inhibiting HPPD, these herbicides inhibit the formation of vitamin E, deriving the plant of one of its antioxidant protective mechanisms.
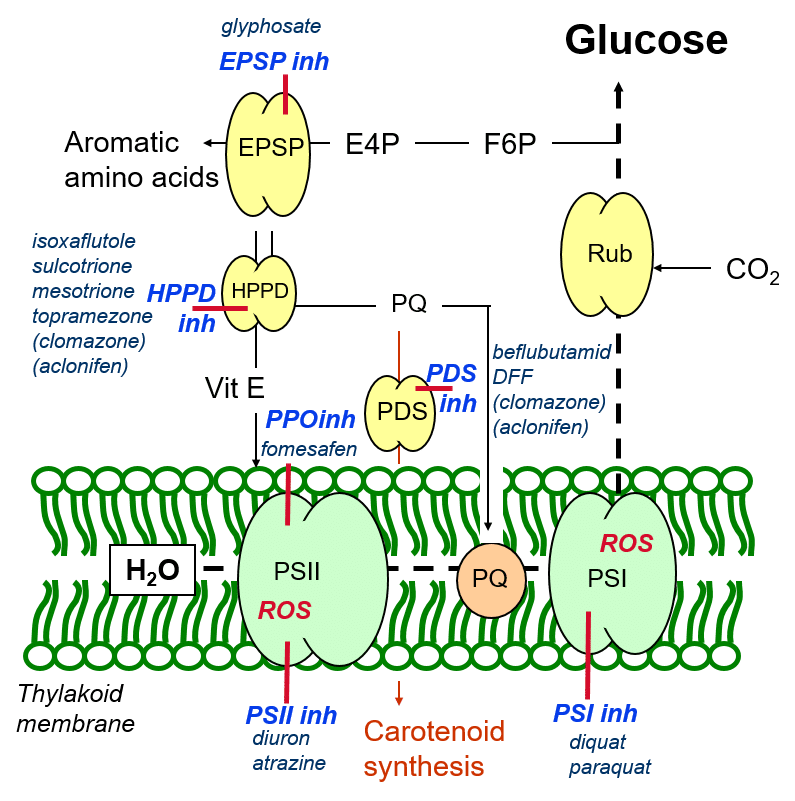
Figure 7: The cascade of herbicide inhibition of aromatic molecule biosynthesis in plants.
In addition, HPPD inhibition results in a reduced production of plastoquinone. In addition to maintaining the redox balance of the photosynthetic apparatus (thereby minimising the creation of oxygen radicals due to over-reduction of the photosystems), plastoquinone is a precursor for the production of aromatic carotenoids, catalysed by the enzyme PDS (phytoene desaturase). Like Vitamin E, carotenoids have a protective antioxidant function through their quenching or scavenging of oxygen radicals.
This herbicidal inhibition of regulatory and antioxidant plant defence mechanisms makes plants susceptible to oxidative damage caused by reactive oxygen species arising from the photosynthetic process. Typical symptoms for HPPD-inhibitory and PDS-inhibitory groups of herbicides include necrotic damage to the leaves due to oxidative damage, and bleaching due to the inhibition of carotenoid biosynthesis, as well as chlorophyll loss due to reduced thylakoid membrane integrity.
PPO inhibitors
Chlorophyll biosynthesis may also be shut down through the herbicidal inhibition of the enzyme PPO (protoporphorinogen oxidase), which catalyses the formation of chlorophyll from precursors. This gives rise to the characteristic bleaching of entire leaves by herbicides of the PPO-inhibitor group.
In the next article in this series, I will discuss additional conventional herbicide and bioherbicide modes of action using the photosynthesis template.
In other articles in this series, I focus on herbicide, fungicide and insecticide modes of action, as well as basic physicochemical and formulation concepts related to pesticide activity.
Harry Teicher is the founder of BIOSCIENCE SOLUTIONS and an Authorpreneur, providing organizations with Strategic- and Project Management as well as Development & Communication solutions. Follow him on Linkedin, Twitter and Facebook.